Figures & data
Magrolimab+bortezomib+dexamethasone may be initiated based on preliminary safety and efficacy data in the magrolimab+carfilzomib+dexamethasone cohort and if initiated, will require only oneprior line of therapy.
aMagrolimab 1 mg/kg initial priming dose then 15–30 mg/kg.
MM: Multiple myeloma; RP2D: Recommended phase II dose; R/R: Relapsed/refractory.
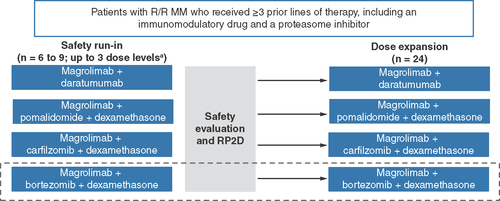
Table 1. Key eligibility criteria.
Table 2. Dosing regimens.
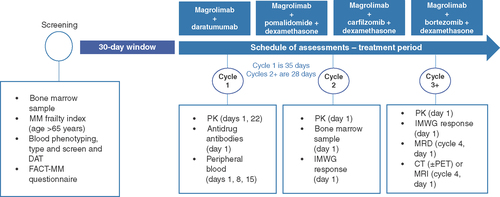
All treatment arms follow the same schedule of assessments. Not exhaustive.
CT: Computed tomography; DAT: Direct antiglobulin test; IMWG: International Myeloma Working Group; MM: Multiple myeloma; PET: Positron emission tomography; PK: Pharmacokinetics.