Figures & data
Figure 1. Mechanism of action of anti-HER2 mAb margetuximab and differences between trastuzumab and margetuximab.
(A) Margetuximab mediates antiproliferative (Fc-independent) and immune activation (Fc-dependent) effects. (B) The Fc engineering of margetuximab alters Fcγ receptor affinities. The red X in panel A (on the left side) indicates inhibition.
ADCC: Antibody-dependent cellular cytotoxicity; ADCP: Antibody-dependent cellular phagocytosis; CD: Cluster of differentiation; Fab: Fragment antigen-binding; Fc: Fragment crystallizable; FcγR: Fragment crystallizable gamma receptor; IgG: Immunoglobin G; M: Margetuximab; mAb: Monoclonal antibody; MHC II: Major histocompatibility complex class II; NK: Natural killer; T: Trastuzumab; TAA: Tumor-associated antigen.
(A) reproduced from [Citation26], with permission from BMJ Publishing Group Ltd.
![Figure 1. Mechanism of action of anti-HER2 mAb margetuximab and differences between trastuzumab and margetuximab.(A) Margetuximab mediates antiproliferative (Fc-independent) and immune activation (Fc-dependent) effects. (B) The Fc engineering of margetuximab alters Fcγ receptor affinities. The red X in panel A (on the left side) indicates inhibition.ADCC: Antibody-dependent cellular cytotoxicity; ADCP: Antibody-dependent cellular phagocytosis; CD: Cluster of differentiation; Fab: Fragment antigen-binding; Fc: Fragment crystallizable; FcγR: Fragment crystallizable gamma receptor; IgG: Immunoglobin G; M: Margetuximab; mAb: Monoclonal antibody; MHC II: Major histocompatibility complex class II; NK: Natural killer; T: Trastuzumab; TAA: Tumor-associated antigen.(A) reproduced from [Citation26], with permission from BMJ Publishing Group Ltd.](/cms/asset/10049847-87f9-4ab4-902e-606c04f7a96c/ifon_a_12333771_f0001.jpg)
Figure 2. SOPHIA progression-free survival (A) and overall survival (B) in the intent-to-treat population.
HR: Hazard ratio; ITT: Intent-to-treat; mo: Month; OS: Overall survival; PFS: Progression-free survival.
(A) Reproduced from [Citation37], with permission from Rugo HS. (B) Reproduced from [Citation19], https://ascopubs.org/journal/jco/.
![Figure 2. SOPHIA progression-free survival (A) and overall survival (B) in the intent-to-treat population.HR: Hazard ratio; ITT: Intent-to-treat; mo: Month; OS: Overall survival; PFS: Progression-free survival.(A) Reproduced from [Citation37], with permission from Rugo HS. (B) Reproduced from [Citation19], https://ascopubs.org/journal/jco/.](/cms/asset/838ec4b2-1481-4475-8420-d4822c65d689/ifon_a_12333771_f0002.jpg)
Table 1. PrespecifiedTable Footnote† exploratory progression-free survival and overall survival subgroup analyses by chemotherapy backbone.
Figure 3. SOPHIA overall survival analysis, per CD16A genotype by treatment group.
(A) Kaplan–Meier estimates of OS by treatment group in CD16A-158F carriers (FF or FV), (B) CD16A-158FF homozygotes, (C) CD16A-158FV heterozygotes and (D) CD16A-158VV homozygotes.
HR: Hazard ratio; OS: Overall survival.
Reproduced from [Citation19], https://ascopubs.org/journal/jco/.
![Figure 3. SOPHIA overall survival analysis, per CD16A genotype by treatment group.(A) Kaplan–Meier estimates of OS by treatment group in CD16A-158F carriers (FF or FV), (B) CD16A-158FF homozygotes, (C) CD16A-158FV heterozygotes and (D) CD16A-158VV homozygotes.HR: Hazard ratio; OS: Overall survival.Reproduced from [Citation19], https://ascopubs.org/journal/jco/.](/cms/asset/cd8252f7-ffdb-4dbf-97df-51028864c76f/ifon_a_12333771_f0003.jpg)
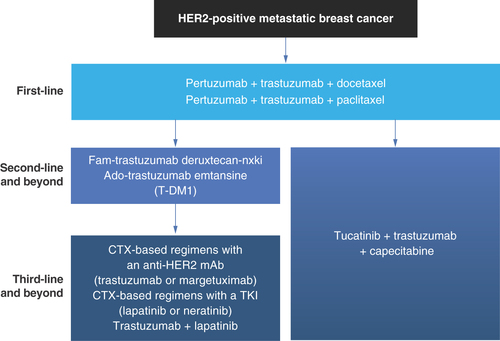
Figure 4. Overall landscape of treatments for HER2-positive metastatic breast cancer in the first-, second- and third-line settings.
CTX: Chemotherapy; mAb: Monoclonal antibody; TKI: Tyrosine kinase inhibitor.