Figures & data
aNSCLC: Advanced or metastatic non-small-cell lung cancer; CS: Corticosteroid; ISD: Immunosuppressive drug; LOT: Line of therapy; NSCLC: Non-small-cell lung cancer.
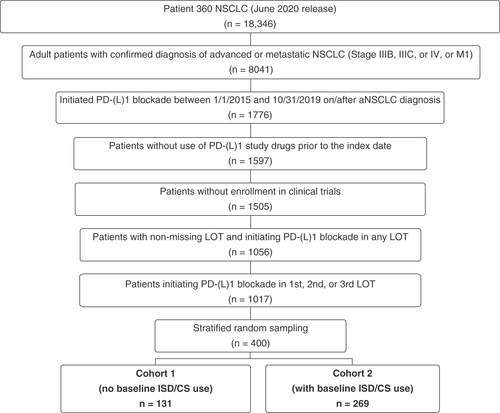
Table 1. Patient characteristics at advanced/metastatic non-small-cell lung cancer diagnosis.
Table 2. Disease characteristics at advanced/metastatic non-small-cell lung cancer diagnosis.
Table 3. PD-(L)1 blockade therapy treatment patterns.
Table 4. Incidence of mild/moderate adverse events of interestTable Footnote† during index PD-(L)1 blockade therapy by category and severity.
Table 5. Management actions performed for the first occurrence of mild/moderate adverse events of interestTable Footnote† after initiation of index PD-(L)1 blockade therapy.
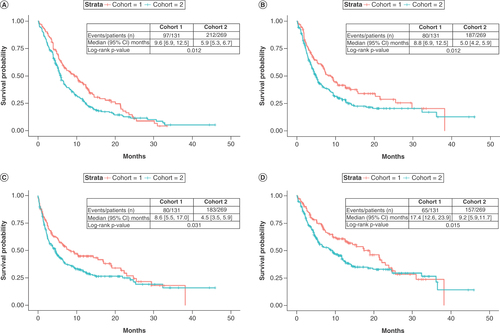
Table 6. Factors associated with overall survival from PD-(L)1 blockade treatment initiation.Table Footnote†
Supplemental material
