Figures & data
Table 1. Cancer-associated proteins that are clients of heat shock protein 90 chaperones.
ATP: Adenosine triphosphate; CDK: Cyclin-dependent kinase; EGFR: Epidermal growth factor receptor; HER2: Human epidermal growth factor receptor 2; HIF-1: Hypoxia-inducible factor 1; HSP 90: Heat shock protein 90; KIT: V-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homologue; PDGFR: Platelet-derived growth factor receptor.
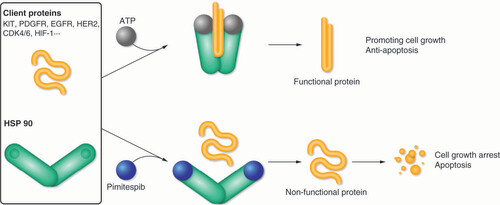
Table 2. Binding affinity of HSP90 inhibitors for proteins in the HSP90 family.
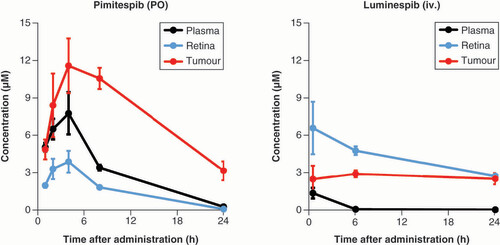
iv.: Intravenously; PO: Orally.