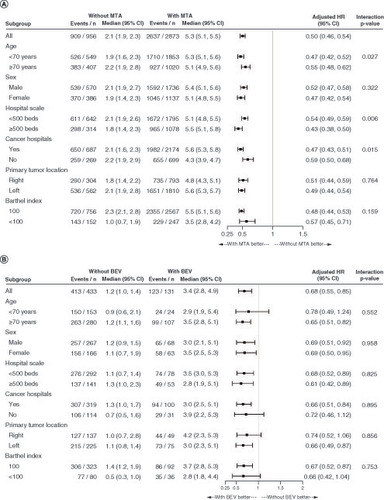
Figures & data
Figure 1. Patient flow chart.
aEGFR: Anti-epidermal growth factor receptor; BEV: Bevacizumab; Cape: Capecitabine; CAPIRI: Capecitabine and irinotecan; CAPOX: Capecitabine and oxaliplatin; CET: Cetuximab; CRC: Colorectal cancer; FOLFIRI: Fluorouracil, leucovorin and irinotecan; FOLFOX: Fluorouracil, leucovorin and oxaliplatin; FOLFOXIRI: Fluorouracil, leucovorin, oxaliplatin and irinotecan; FP: Fluoropyrimidine; 5-FU: Fluorouracil; IRI: Irinotecan; IRIS: S-1 and irinotecan; IT: Intensive therapy; JSCCR: Japanese Society for Cancer of the Colon and Rectum; LIT: Less intensive therapy; l-LV: l-Leucovorin; LV: Leucovorin; mCRC: Metastatic colorectal cancer; OX: Oxaliplatin; PANI: Panitumumab; S-1: Tegafur/gimeracil/oteracil potassium; SOX: S-1 and oxaliplatin; UFT: Tegafur/uracil.
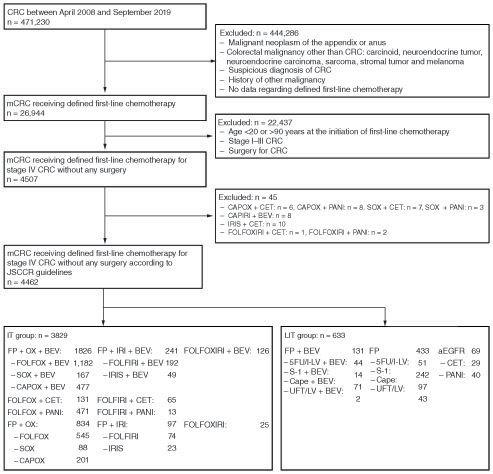
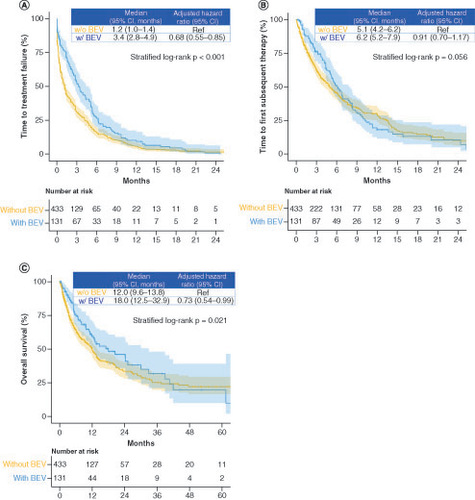
Figure 2. Subgroup analysis of time to treatment failure between the intensive and less intensive therapy groups.
HR: Hazard ratio; IT: Intensive therapy; LIT: Less intensive therapy.
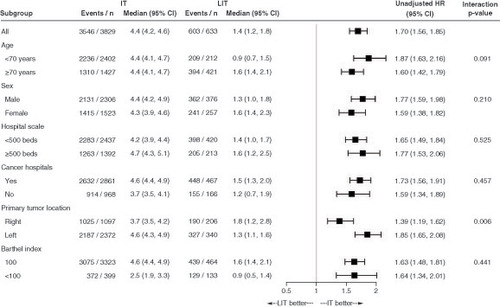
Table 1. Patient characteristics.
Figure 3. Time to treatment failure, time to first subsequent therapy and overall survival in the intensive and less intensive therapy groups.
Kaplan–Meier curves of (A) time to treatment failure, (B) time to first subsequent therapy and (C) overall survival.
IT: Intensive therapy; LIT: Less intensive therapy.
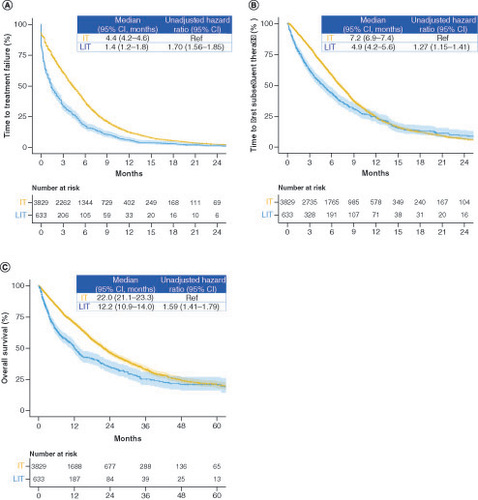
Figure 4. Time to treatment failure, time to first subsequent therapy and overall survival in the groups receiving treatment with and without molecularly targeted agents.
Kaplan-Meier curves of (A) time to treatment failure, (B) time to first subsequent therapy and (C) overall survival.
MTA: Molecularly targeted agent; w/: With; w/o: Without.
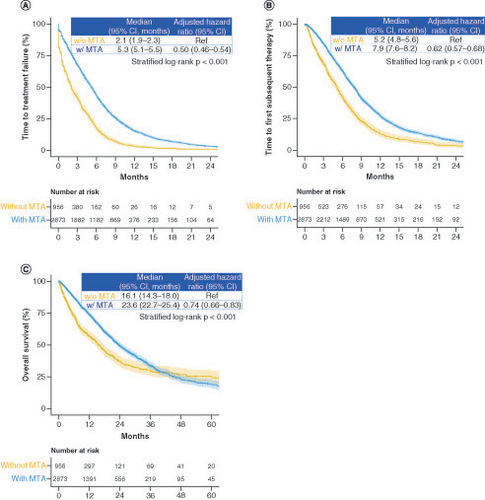
Table S1. Patient characteristics and Barthel indexscores
Download MS Word (29.5 KB)Table S2. Patient characteristics in the with- and without-molecularly-targeted agent (MTA)subgroups.
Download MS Word (32.3 KB)Table S3. Patient characteristics in the IT (intensive therapy) and LIT(less-intensive therapy) subgroups.
Download MS Word (32.2 KB)Supplementary Material
Download TIFF Image (852.2 KB)Supplementary Material
Download TIFF Image (912.9 KB)Supplementary Material
Download TIFF Image (873.4 KB)Data sharing statement
Data generated and/or analyzed during this study will not be shared due to concerns about data protection regulations in our country.