Figures & data
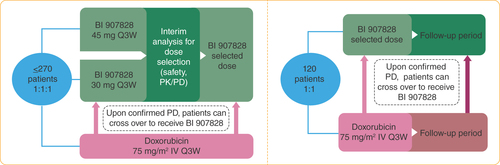
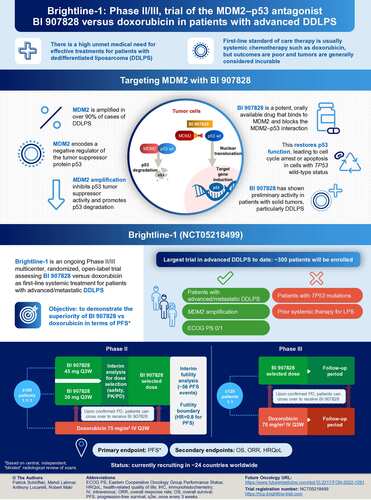
Patients may continue treatment with doxorubicin for up to six cycles (maximum cumulative dose of 450 mg/m2); patients randomized to the doxorubicin arm will be eligible to cross-over to receive BI 907828 following confirmed progressive disease and if eligibility criteria are met.
IV: Intravenous; PD: Pharmacodynamic; PFS: Progression-free survival; PK: Pharmacokinetic; Q3W: Every 3 weeks.