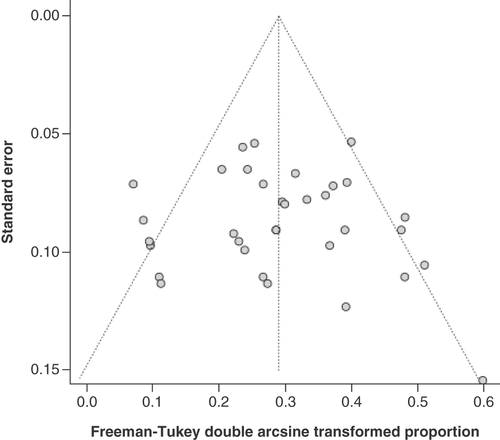
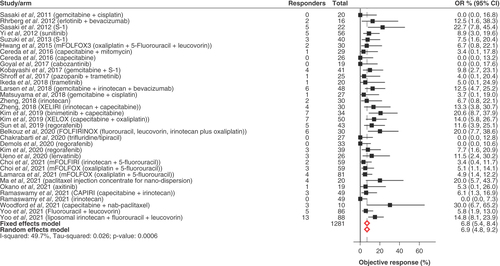
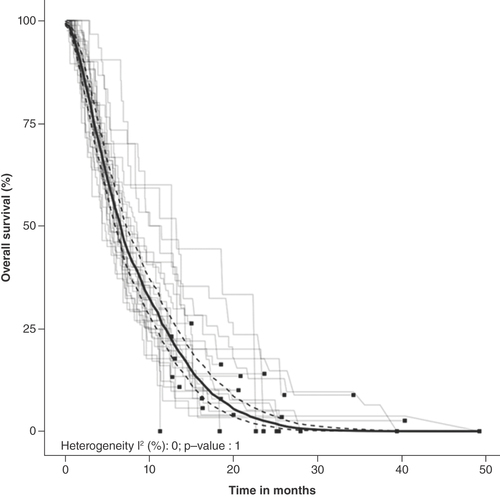
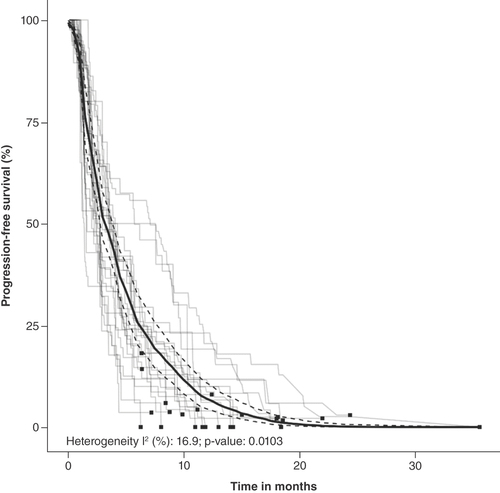
Figures & data
Table 1.
PICOST eligibility criteria for the systematic review.
Table 2.
General characteristics of studies included in the systematic review.
Table 3.
Summary of pooled Kaplan–Meier estimates of overall survival and progression-free survival.