Figures & data
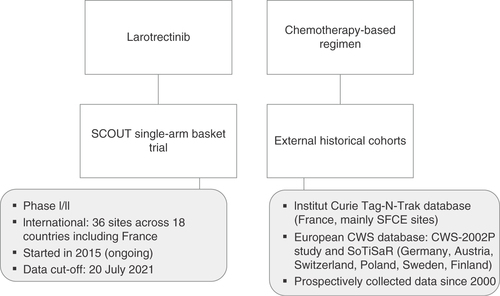
Table 1. Selection criteria of data sources for the external historical control arm.
CWS: Cooperative Weichteilsarkom Studiengruppe; SFCE: Société Française de lutte contre les Cancers et les leucémies de l’Enfant et de l’adolescent; SoTiSaR: Soft Tissue Sarcoma Registry.