Figures & data
(A) Design of datopotamab deruxtecan monoclonal antibody and (B) proposed mechanism of action of datopotamab deruxtecan.
Panel A previously presented at the 2019 World Conference on Lung Cancer [Citation32]. Panel B reproduced with kind permission of the authors with permission from [Citation33].
DXd: Deruxtecan; IgG1: Immunoglobulin G1; mAb: Monoclonal antibody; TROP2: Trophoblast cell surface antigen 2.
![Figure 1. Overview of the monoclonal antibody datopotamab deruxtecan. (A) Design of datopotamab deruxtecan monoclonal antibody and (B) proposed mechanism of action of datopotamab deruxtecan.Panel A previously presented at the 2019 World Conference on Lung Cancer [Citation32]. Panel B reproduced with kind permission of the authors with permission from [Citation33].DXd: Deruxtecan; IgG1: Immunoglobulin G1; mAb: Monoclonal antibody; TROP2: Trophoblast cell surface antigen 2.](/cms/asset/285463d0-b7e5-44c0-a6d3-35cb164e6f55/ifon_a_12333896_f0001.gif)
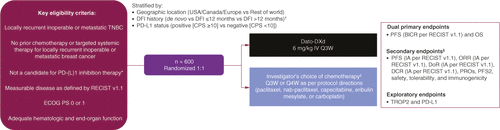
Table 1. Study interventions.
*PD-L1 negative, previous PD-1/PD-L1 inhibitor therapy for early-stage breast cancer, comorbidities precluding PD-1/PD-L1 inhibitor therapy, or PD-L1 positive with no regulatory access to PD-1/PD-L1 inhibitor therapy (no regulatory approval in country).
†DFI defined as the time between completion of treatment with curative intent (either surgery or last dose of systemic anticancer therapy) and first documented local or distant disease recurrence. Adjuvant radiation therapy is not considered treatment with curative intent for the purpose of calculating. No minimum DFI was specified; patients could have had any length of time between curative-intent treatment and disease recurrence.
‡If no prior taxane, or prior taxane in the (neo)adjuvant setting and DFI >12 months, paclitaxel or nab-paclitaxel; if prior taxane and DFI ≤12 months: capecitabine, carboplatin, or eribulin mesylate.
§Response assessment: scan Q6W for 48 weeks, then Q9W until RECIST v1.1, regardless of study intervention, discontinuation or start of subsequent anticancer therapy.
BICR: Blinded independent central review; CPS: Combined positive score; Dato-DXd: Datopotamab deruxtecan; DCR: Disease control rate; DFI: Disease-free interval; DoR: Duration of response; ECOG PS: Eastern Cooperative Oncology Group Performance Status; IA: Investigator’s assessment; iv.: Intravenous; ORR: Objective response rate; OS: Overall survival; PD-1: Programmed cell death protein-1; PD-L1: Programmed cell death-ligand 1; PFS: Progression-free survival; PFS2: Time to second progression or death; PRO: Patient-reported outcome; Q3/4/6/9W: Every 3/4/6/9 weeks; RECIST v1.1: Response Evaluation Criteria in Solid Tumours version 1.1; TNBC: Triple-negative breast cancer; TROP2: Trophoblast cell surface protein 2.