Figures & data
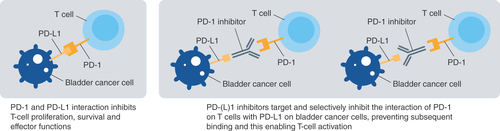
PD-1: Programmed cell death-1; PD-L1: Programmed cell death-ligand 1.
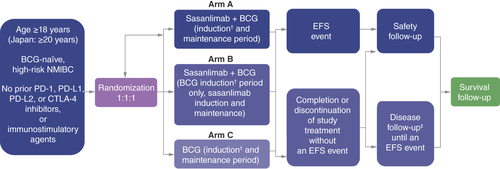
CREST is a phase III study evaluating the efficacy and safety of sasanlimab in combination with BCG versus BCG monotherapy for patients with BCG-naive high-risk NMIBC. EFS is defined as the time from randomization to date of the first EFS event.
†Re-induction is permitted for participants with CIS at randomization who have persistent disease at 3 months after initiating study treatment, or participants who have recurrence of high-grade Ta disease at 3 months after initiating study treatment (TURBT is required before re-induction).
‡Only participants who complete treatment with no evidence of disease progression or recurrence proceed to disease follow-up.
BCG: Bacillus Calmette-Guérin; CIS: Carcinoma in situ; CTLA-4: Cytotoxic T lymphocyte-associated antigen 4; EFS: Event-free survival; NMIBC: Non-muscle-invasive bladder cancer; PD-1: Programmed cell death-1; PD-L1/PD-L2: Programmed cell death-ligand 1/2; Ta: Stage of bladder cancer defined as a non-invasive papillary carcinoma; TURBT: Transurethral resection of bladder tumor.
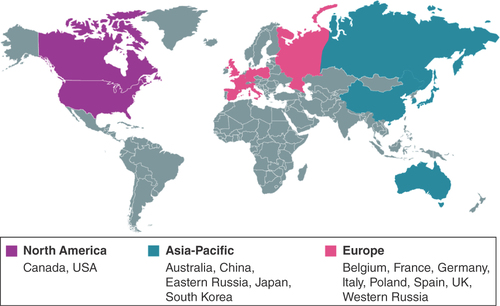
CREST’s target sample size is approximately 1000 patients across centers in Asia, Australia, Europe and North America.
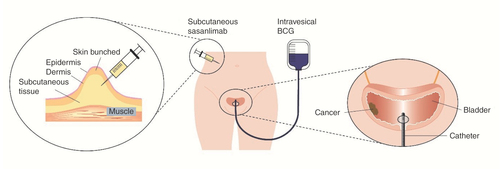
Sasanlimab is administered by subcutaneous injection into the adipose tissue layer below the epidermis and dermis. BCG is administered by intravesical instillation directly into the bladder via a urinary catheter.
BCG: Bacillus Calmette-Guérin.