Figures & data
Figure 1. Study concept and design.
(A) Depicts how the analysis isolates the impact of the AA pathway on patient survival. All patients start with the initial treatment (a new drug with extended survival) and then surviving patients progress to a subsequent line of therapy. As the AA drug represents the best overall survival, it is assumed that more patients who are alive when the drug is approved will use the AA drug, and the remainder of patients are distributed between the no treatment C and SoC B cohorts based on real-world trends in the real-word data. As the timing of the AA drug availability is delayed for the counterfactual scenario, the percentage of patients alive and eligible for the drug reduces. The analysis compares average patient outcomes across these two possible outcomes to isolate how the timing of the AA drug impacts patient outcomes. (B) Depicts how patients flow through initial and subsequent treatments across the three treatment cohorts (AA drug [A], SoC [B], no treatment [C]). Each cohort is followed up from the time of the initiation of the initial drug through subsequent treatments to estimate overall survival at the patient level before extrapolating patient-level outcomes to population estimates based on real-world market use of AA drugs during the AA period based on shares from publicly available epidemiology data.
*Next-line SoC treatments for surviving patients in cohorts A and B may occur in pre-AA period.
AA: Accelerated approval; SoC: Standard of care.
![Figure 1. Study concept and design.(A) Depicts how the analysis isolates the impact of the AA pathway on patient survival. All patients start with the initial treatment (a new drug with extended survival) and then surviving patients progress to a subsequent line of therapy. As the AA drug represents the best overall survival, it is assumed that more patients who are alive when the drug is approved will use the AA drug, and the remainder of patients are distributed between the no treatment C and SoC B cohorts based on real-world trends in the real-word data. As the timing of the AA drug availability is delayed for the counterfactual scenario, the percentage of patients alive and eligible for the drug reduces. The analysis compares average patient outcomes across these two possible outcomes to isolate how the timing of the AA drug impacts patient outcomes. (B) Depicts how patients flow through initial and subsequent treatments across the three treatment cohorts (AA drug [A], SoC [B], no treatment [C]). Each cohort is followed up from the time of the initiation of the initial drug through subsequent treatments to estimate overall survival at the patient level before extrapolating patient-level outcomes to population estimates based on real-world market use of AA drugs during the AA period based on shares from publicly available epidemiology data.*Next-line SoC treatments for surviving patients in cohorts A and B may occur in pre-AA period.AA: Accelerated approval; SoC: Standard of care.](/cms/asset/084f3709-e87c-44f4-8c1c-e63e443f27be/ifon_a_2337507_f0001_c.jpg)
Table 1. Included drugs and key approval dates.
Table 2. Real-world outcomes: proportion of patients receiving AA drugs and mean survival gains for patients.
Table 3. Population outcomes for AA pathway compared with counterfactual scenarios.
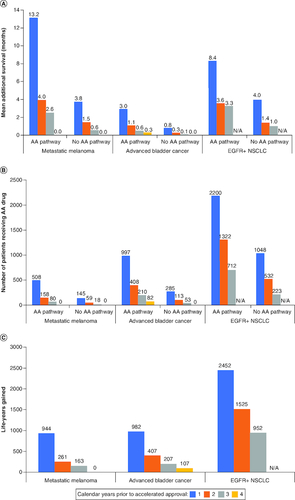
Figure 2. Accelerated approval pathway outcomes.
(A) Average additional survival (in months) per patient with and without AA pathway by proximity to the AA date; (B) number of patients receiving AA drug with and without AA pathway by proximity to the AA date; and (C) LYs gained for initial drug patients with AA pathway by proximity to the AA date.
The calendar year prior to AA reflects the year of initial drug start relative to the date of the AA drug.
AA: Accelerated approval; EGFR+: Epidermal growth factor receptor positive; LYs: Life years; N/A: Not applicable; NSCLC: Non-small-cell lung cancer.