Figures & data
(A) Dual cytotoxic mechanisms of PARP inhibitors; (B) PARP inhibitor in combination with an ARSI.
Adapted with permission from Future Oncology [Citation29]. Reprinted (or adapted) from Cancer Research [Citation30].
AR: Androgen receptor; ARSI: Androgen-receptor signaling inhibitor; DDR: DNA damage response; DSB: Double-strand break; HRD: Homologous recombination deficiency; PARP: Poly(ADP-ribose) polymerase; PARPi: Poly(ADP-ribose) polymerase inhibitor; SSB: Single-strand break.
![Figure 1. Mechanistic overview of PARP inhibition. (A) Dual cytotoxic mechanisms of PARP inhibitors; (B) PARP inhibitor in combination with an ARSI.Adapted with permission from Future Oncology [Citation29]. Reprinted (or adapted) from Cancer Research [Citation30].AR: Androgen receptor; ARSI: Androgen-receptor signaling inhibitor; DDR: DNA damage response; DSB: Double-strand break; HRD: Homologous recombination deficiency; PARP: Poly(ADP-ribose) polymerase; PARPi: Poly(ADP-ribose) polymerase inhibitor; SSB: Single-strand break.](/cms/asset/5163bfeb-3e58-447e-8e10-90bbdbbdca5f/ifon_a_12367119_f0001.jpg)
*No prior life-prolonging systemic therapy for castration-sensitive prostate cancer, excluding ≤3 months androgen deprivation therapy with or without an approved androgen-receptor signaling inhibitor; treatment with estrogens, cyproterone acetate, or first-generation anti-androgens is allowed until randomization.
†No prior life-prolonging systemic therapy for castration-resistant prostate cancer, excluding androgen deprivation therapy and first-generation antiandrogens.
mCRPC: Metastatic castration-resistant prostate cancer; mCSPC: Metastatic castration-sensitive prostate cancer; nmCRPC: Non-metastatic castration-resistant prostate cancer.
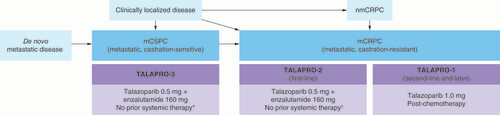
Table 1. Key inclusion and exclusion criteria of the TALAPRO-3 study.
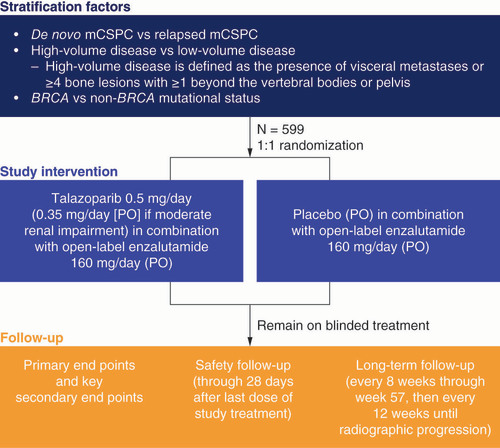
Table 2. TALAPRO-3 study end points.
Supplementary Data 1
Download PDF (358.8 KB)Data sharing statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.