Figures & data
Table 1.
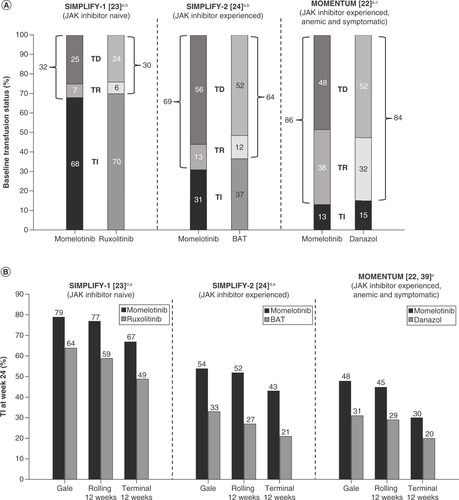
Anemia-related response end points defined by transfusion status or hemoglobin improvement applied across clinical trials of myelofibrosis to date.