Figures & data
During fSCIG treatment, individual patients were enrolled and some patients had medication changed or discontinued. The boxes in the middle indicate study time points (start of the study, transition to the next quarter [Q], end of the study).
fSCIG: Hyaluronidase-facilitated subcutaneous immunoglobulin; Ig20Gly: Subcutaneous immunoglobulin 20%.
![Figure 1. Flow of pediatric patients with primary immunodeficiency diseases receiving hyaluronidase-facilitated subcutaneous immunoglobulin.During fSCIG treatment, individual patients were enrolled and some patients had medication changed or discontinued. The boxes in the middle indicate study time points (start of the study, transition to the next quarter [Q], end of the study).fSCIG: Hyaluronidase-facilitated subcutaneous immunoglobulin; Ig20Gly: Subcutaneous immunoglobulin 20%.](/cms/asset/4f8d738c-1884-46e4-a20e-a9c6b6c037bb/iimy_a_12366985_f0001.jpg)
Table 1. Ramp-up schedule of fSCIG in pediatric patients with primary immunodeficiency diseases.
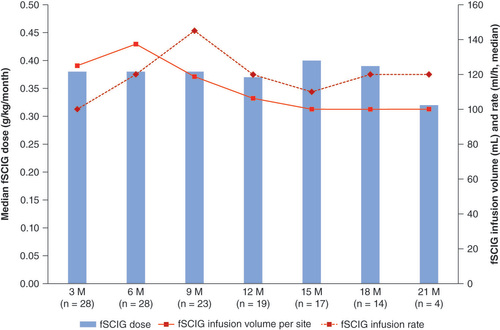
Table 2. Treatment parameters for fSCIG in pediatric patients with primary immunodeficiency diseases.
The number of patients administering hyaluronidase-facilitated subcutaneous immunoglobulin during the given treatment period is indicated on the x-axis.
M: Month.
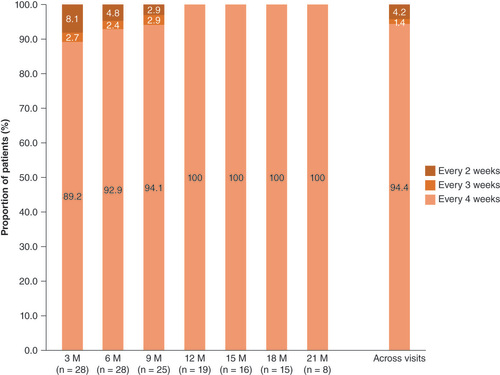
fSCIG: Hyaluronidase-facilitated subcutaneous immunoglobulin; M: Month.