Figures & data
Table 1.
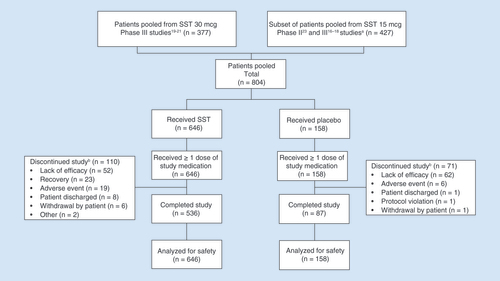
Patient study pools for safety analysis.
Table 2.
Patient demographics and baseline characteristics in the sufentanil sublingual tablet clinical studies (pooled).
Table 3.
Adverse events in the sufentanil sublingual tablet clinical studies (pooled): all adverse events and adverse events related† to study treatment.
Table 4.
Serious adverse events.
Table 5.
Higher versus lower 24-h dosing of sufentanil sublingual tablet in Phase III studies ≥24 h: lowest oxygen saturation by treatment and dose group.
Table 6.
Median drug utilization: morphine equivalent per sufentanil sublingual tablet dose based on duration of exposure.
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of this shared data is in accordance with the terms (if any) agreed upon their receipt. The source of this data is: Clinicaltrials.gov identifiers: NCT02356588, NCT02662556, NCT02447848, NCT00859313, NCT00612534, NCT00718081, NCT01539538, NCT01539642 and NCT01660763.