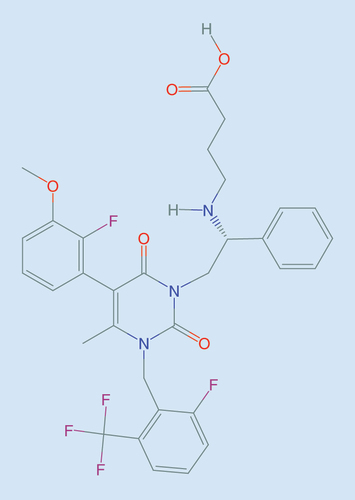
Figures & data
The figures in the upper level present the interaction of native GnRH (A), depot GnRH agonists (B) and GnRH antagonists (C) with the GnRH-R in the pituitary gland. The corresponding figures in the lower level present relative estradiol suppression. (A) Native GnRH is released in a pulsatile manner from the hypothalamus. The frequency of GnRH pulses regulates cyclic changes in gonadotropins (LH, FSH) and estradiol concentrations during the cycle. (B) Depot GnRH agonists initially stimulate the HPO axis, which results in a transient hormonal flare. Continued stimulation of GnRH-R consequently leads to their desensitization and profound suppression and profound estradiol suppression. (C) GnRH antagonists competitively bind to GnRH-R and produce rapid and dose-dependent suppression of the HPO axis, which results in a partial estradiol suppression at lower doses to nearly full suppression at higher doses.
FSH: Follicle-stimulating hormone; GnRH: Gonadotropin-releasing hormone; GnRH-R: GnRH receptor; HPO: Hypothalamo–pituitary–ovarian; LH: Luteinizing hormone.
Concentrations (means + SD) of FSH (A), LH (B), E2 (C) and P (D) during 21 days of dosing with placebo or elagolix. For the placebo group, the error bars for some time points have been truncated.
BID: Twice daily; E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; P: Progesterone; QD: Once daily; SD: Standard deviation.
Reprinted with permission from [Citation84].
![Figure 3. Dose-dependent suppression of gonadotropins (LH, FSH), E2, and progesterone in healthy premenopausal women.Concentrations (means + SD) of FSH (A), LH (B), E2 (C) and P (D) during 21 days of dosing with placebo or elagolix. For the placebo group, the error bars for some time points have been truncated.BID: Twice daily; E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; P: Progesterone; QD: Once daily; SD: Standard deviation.Reprinted with permission from [Citation84].](/cms/asset/e4e31655-8ac0-4dd9-a3db-90354b12cbe4/ipmt_a_12345123_f0003.jpg)
Shown are the percentages of women in whom the two primary end points (clinically meaningful reduction in dysmenorrhea or in NMPP, as measured by the decreased or stable use of rescue analgesic agents) were reported at 3 and 6 months in Elaris EM-I (A) and Elaris EM-II (B). In Elaris EM-I, 3-month data are provided for 373 women who received placebo, 248 who received the lower elagolix dose (150 mg QD) and 244 who received the higher elagolix dose (200 mg BID); the corresponding 6-month data are provided for 372, 247 and 243 women. In Elaris EM-II, 3-month data are provided for 353 women who received placebo, 221 who received the lower elagolix dose and 225 who received the higher elagolix dose; the corresponding 6-month data are provided for 355, 221 and 225 women.
BID: Twice daily; EM: Endometriosis; NMPP: Nonmenstrual pelvic pain; QD: Once daily.
Reprinted with permission from [Citation94] © Massachusetts Medical Society (2017).
![Figure 4. Reduction in dysmenorrhea and nonmenstrual pelvic pain during treatment with elagolix in women with endometriosis-associated pain (Elaris EM-I and EM-II studies).Shown are the percentages of women in whom the two primary end points (clinically meaningful reduction in dysmenorrhea or in NMPP, as measured by the decreased or stable use of rescue analgesic agents) were reported at 3 and 6 months in Elaris EM-I (A) and Elaris EM-II (B). In Elaris EM-I, 3-month data are provided for 373 women who received placebo, 248 who received the lower elagolix dose (150 mg QD) and 244 who received the higher elagolix dose (200 mg BID); the corresponding 6-month data are provided for 372, 247 and 243 women. In Elaris EM-II, 3-month data are provided for 353 women who received placebo, 221 who received the lower elagolix dose and 225 who received the higher elagolix dose; the corresponding 6-month data are provided for 355, 221 and 225 women.BID: Twice daily; EM: Endometriosis; NMPP: Nonmenstrual pelvic pain; QD: Once daily.Reprinted with permission from [Citation94] © Massachusetts Medical Society (2017).](/cms/asset/711c2aa5-d0a9-4f45-b53e-c8cc80d7db4e/ipmt_a_12345123_f0004.jpg)
Statistical significance compared with placebo for the mean percentage change from baseline score in Elaris EM-I (A) and Elaris EM-II (B) was based on a mixed-effects model with repeated measures with treatment as the main effect, visit as the repeated measure, baseline value as a covariate and an interaction between treatment and visit, using observed data, indicated by: *p < 0.05, **p < 0.01; ***p ≤ 0.001. Error bars represent standard error.
EM: Endometriosis; LS: Least squares.
Reprinted with permission from [Citation94] © Massachusetts Medical Society (2017).
![Figure 5. Mean percentage change from baseline in dysmenorrhea and nonmenstrual pelvic pain scores during treatment with elagolix in women with endometriosis-associated pain (Elaris EM-I and EM-II studies).Statistical significance compared with placebo for the mean percentage change from baseline score in Elaris EM-I (A) and Elaris EM-II (B) was based on a mixed-effects model with repeated measures with treatment as the main effect, visit as the repeated measure, baseline value as a covariate and an interaction between treatment and visit, using observed data, indicated by: *p < 0.05, **p < 0.01; ***p ≤ 0.001. Error bars represent standard error.EM: Endometriosis; LS: Least squares.Reprinted with permission from [Citation94] © Massachusetts Medical Society (2017).](/cms/asset/8868a4c5-dbc7-4d6d-a08f-ee518f60f5c6/ipmt_a_12345123_f0005.jpg)
Mean percentage change from baseline in dysmenorrhea (A & D), nonmenstrual pelvic pain (B & E) and dyspareunia scores (C & F) during long-term treatment with elagolix in women with endometriosis-associated pain (Elaris EM-III and EM-IV extension studies). Error bars represent 95% CIs. Between-group comparisons were not predefined and not performed. Months 1–6 in Elaris EM-I and Elaris EM-II are from women who enrolled in the extension studies.
BID: Twice daily; EM: Endometriosis; QD: Once daily.
Reprinted with permission from [Citation95]. https://journals.lww.com/greenjournal/
![Figure 6. Mean percentage change from baseline.Mean percentage change from baseline in dysmenorrhea (A & D), nonmenstrual pelvic pain (B & E) and dyspareunia scores (C & F) during long-term treatment with elagolix in women with endometriosis-associated pain (Elaris EM-III and EM-IV extension studies). Error bars represent 95% CIs. Between-group comparisons were not predefined and not performed. Months 1–6 in Elaris EM-I and Elaris EM-II are from women who enrolled in the extension studies.BID: Twice daily; EM: Endometriosis; QD: Once daily.Reprinted with permission from [Citation95]. https://journals.lww.com/greenjournal/](/cms/asset/15be4fac-de19-408c-8228-12035dca5755/ipmt_a_12345123_f0006.jpg)