Figures & data
Peripheral nerve damage and following neuroinflammatory events cause intense nonphysiological pain signals to spinal dorsal horn neurons, in which cPLA2 and iPLA2 are activated, followed by a production of enough amounts of LPC. Some aliquots of LPC released from stimulated neurons are converted to LPA by autotaxin (lysophospholipase D), Thus, produced LPA in turn activates microglia and produces cytokines, such as IL-1β, which activates neuron and stimulates cPLA2 and iPLA2. and presumably also secretory sPLA2. Thus, the initial intense nonphysiological activation of spinal dorsal horn neurons may lead to a feed-forward LPA production, and possibly to a cause of chronic pain. LPA also goes back to DR fibers and DRG, where LPA1-mediated demyelination underlying allodynia and upregulation of Cavα2δ1 and ephrin B1 underlying hyperalgesia are caused. These mechanisms also contribute to the functional feed-forward system of pain transmission. On the other hand, LPA has an action of BDNF production in microglia through an activation of P2X4 receptor by secreted ATP. As BDNF is known to decrease neuronal plasma membrane KCC2 levels and increase cytosolic Cl- ion levels, resulting in a conversion of GABAA receptor function from inhibitory to excitatory one. All these mechanisms may play roles in the development of neuropathic pain [Citation24]. Astrocyte activation, on the other hand, may be also involved in the maintenance of neuropathic pain through a production of chemokines [Citation41].
DRG: Dorsal root ganglion; LPA: Lysophosphatidic acid; LPC: Lysophosphatidyl choline.
![Figure 1. Feed-forward LPA-mediated mechanisms in neuropathic pain following nerve damage or neuroinflammation.Peripheral nerve damage and following neuroinflammatory events cause intense nonphysiological pain signals to spinal dorsal horn neurons, in which cPLA2 and iPLA2 are activated, followed by a production of enough amounts of LPC. Some aliquots of LPC released from stimulated neurons are converted to LPA by autotaxin (lysophospholipase D), Thus, produced LPA in turn activates microglia and produces cytokines, such as IL-1β, which activates neuron and stimulates cPLA2 and iPLA2. and presumably also secretory sPLA2. Thus, the initial intense nonphysiological activation of spinal dorsal horn neurons may lead to a feed-forward LPA production, and possibly to a cause of chronic pain. LPA also goes back to DR fibers and DRG, where LPA1-mediated demyelination underlying allodynia and upregulation of Cavα2δ1 and ephrin B1 underlying hyperalgesia are caused. These mechanisms also contribute to the functional feed-forward system of pain transmission. On the other hand, LPA has an action of BDNF production in microglia through an activation of P2X4 receptor by secreted ATP. As BDNF is known to decrease neuronal plasma membrane KCC2 levels and increase cytosolic Cl- ion levels, resulting in a conversion of GABAA receptor function from inhibitory to excitatory one. All these mechanisms may play roles in the development of neuropathic pain [Citation24]. Astrocyte activation, on the other hand, may be also involved in the maintenance of neuropathic pain through a production of chemokines [Citation41].DRG: Dorsal root ganglion; LPA: Lysophosphatidic acid; LPC: Lysophosphatidyl choline.](/cms/asset/0bfc8f01-8dfd-4f31-926d-9b157bec052a/ipmt_a_12344353_f0001.jpg)
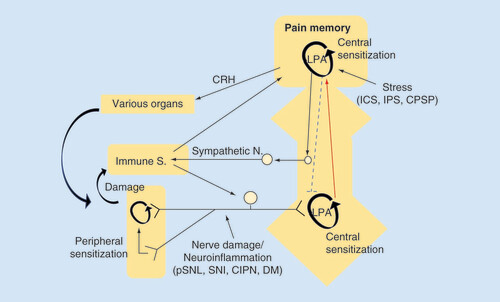
In the peripheral NeuP model, nerve damage or neuroinflammation causes LPA production in the spinal dorsal horn, where LPA production is self-amplified and LPA-mediated pain memory is developed and maintained in a feed-forward manner. In the centralized pain, represented by fibromyalgia, various types of intense stress experience develop and maintain the LPA receptor-mediated pain memory. Although conclusive data, including LPA-involvement, are not yet available, the brain plasticity related to pain memory may attenuate the descending pain-inhibitory system and activate peripheral immune system, which in turn reinforces the brain pain memory through the peripheral pain pathway.
CIPN: Chemotherapy-induced peripheral neuropathy; CPSP: Central post-stroke pain; CRH: Corticotropin releasing hormone; DM: Diabetes mellitus; ICS: Intermittent cold stress; IPS: Intermittent psychological stress; LPA: Lysophosphatidic acid; pSNL: Partial sciatic nerve ligation; SNI: Spare nerve injury.