Figures & data
Table 1. Comparison of the buprenorphine transdermal system and buprenorphine buccal film.
Buprenorphine buccal film is a small, bilayered dissolving polymer film that adheres to the buccal mucosa using BioErodible MucoAdhesive® technology, which enhances bioavailability compared with other routes of administration [Citation59]. Buprenorphine buccal film is available in 75, 150, 300, 450, 600, 750 and 900 μg dosage strengths and facilitates a unidirectional flow of the drug that is approximately 46–65% bioavailable and reaches steady-state levels in approximately 3 days [Citation50,Citation54].
![Figure 1. Buprenorphine buccal film. Buprenorphine buccal film is a small, bilayered dissolving polymer film that adheres to the buccal mucosa using BioErodible MucoAdhesive® technology, which enhances bioavailability compared with other routes of administration [Citation59]. Buprenorphine buccal film is available in 75, 150, 300, 450, 600, 750 and 900 μg dosage strengths and facilitates a unidirectional flow of the drug that is approximately 46–65% bioavailable and reaches steady-state levels in approximately 3 days [Citation50,Citation54].](/cms/asset/a1412f9f-893f-483e-9211-0682168a2915/ipmt_a_12344370_f0001.jpg)
The safety and efficacy of buprenorphine buccal film were studied in two double-blind, Phase III, randomized, placebo-controlled trials in (A) opioid-experienced [Citation44] and (B) opioid-naive [Citation46] patients that comprised a 2-week screening phase; an open-label titration phase; a 12-week double-blind, placebo-controlled treatment phase; and follow-up. An analgesic taper phase was also included for opioid-experienced patients. (C) The long-term study [Citation45] included rollover patients from (A & B) or de novo recruits and was comprised of a dose-titration period of ≤6 weeks with buprenorphine buccal film; patients who achieved and maintained an optimal dose (300–900 μg/12 h) for ≥7 days continued treatment for a long-term period up to 48 weeks.
![Figure 2. Design of buprenorphine buccal film clinical trials in patients with chronic low back pain. The safety and efficacy of buprenorphine buccal film were studied in two double-blind, Phase III, randomized, placebo-controlled trials in (A) opioid-experienced [Citation44] and (B) opioid-naive [Citation46] patients that comprised a 2-week screening phase; an open-label titration phase; a 12-week double-blind, placebo-controlled treatment phase; and follow-up. An analgesic taper phase was also included for opioid-experienced patients. (C) The long-term study [Citation45] included rollover patients from (A & B) or de novo recruits and was comprised of a dose-titration period of ≤6 weeks with buprenorphine buccal film; patients who achieved and maintained an optimal dose (300–900 μg/12 h) for ≥7 days continued treatment for a long-term period up to 48 weeks.](/cms/asset/4a4fd90f-cf4c-46da-afa2-f21a326f71b5/ipmt_a_12344370_f0002.jpg)
Primary sensitivity analyses comparing the change from baseline to week 12 in numerical rating scale pain intensity scores in patients receiving placebo compared with those receiving buprenorphine buccal film.
LCL: Lower confidence limit; UCL: Upper confidence limit.
Data taken from [Citation44,Citation46].
![Figure 3. Double-blind studies: buprenorphine buccal film significantly reduced pain intensity scores compared with placebo in patients with chronic low back pain. Primary sensitivity analyses comparing the change from baseline to week 12 in numerical rating scale pain intensity scores in patients receiving placebo compared with those receiving buprenorphine buccal film.LCL: Lower confidence limit; UCL: Upper confidence limit.Data taken from [Citation44,Citation46].](/cms/asset/1ca00dba-4551-4039-a6ef-daaf39c3d3b5/ipmt_a_12344370_f0003.jpg)
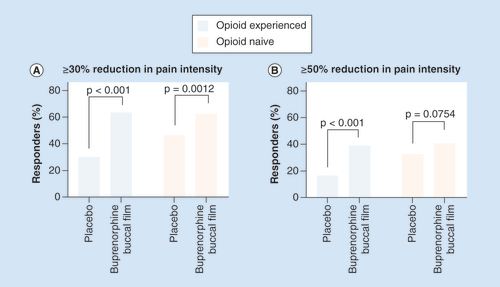
The percentage of patients achieving ≥30% (A) or ≥50% (B) pain reduction from the period before the open-label titration to week 12 of the double-blind treatment phase.