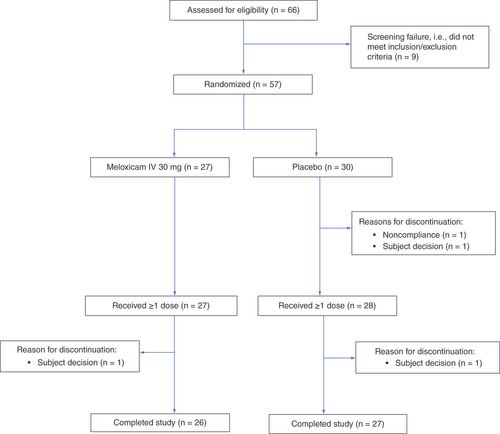
Figures & data
Table 1. Enhanced recovery after surgery protocol.
Table 2. Subject demographics, baseline characteristics and surgical characteristics.
Table 3. Adverse events occurring in ≥2 subjects in either study group.
Table 4. Adverse events of special interest occurring in ≥1 subject in either study group.
Table 5. Summary of total opioid consumption (in mg of IV morphine equivalent dose) by time interval.
Table 6. Economic and healthcare resource data.
Supplemental Data 1
Download Zip (23.5 KB)Data sharing statement
The authors certify that this manuscript reports original clinical trial data, NCT03323385. Individual, de-identified participant data will not be available. Data will be available to the public at https://clinicaltrials.gov/ct2/show/NCT03323385 for the duration of 2020.