Figures & data
Treatment period of 15 weeks comprising a 3-week titration period and a 12-week maintenance period. Starting dose was 100 mg tapentadol PR in all patients. Analysis of patients in the ITT who entered the maintenance period.
ITT: Intent to treat set; mITT: ITT set who entered maintenance period.
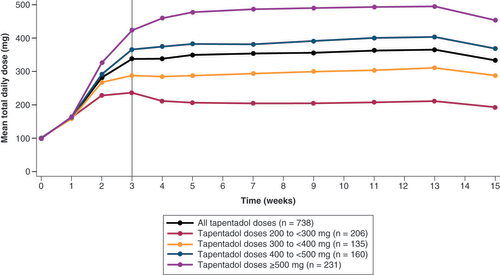
Table 1. Subject demographics categorized by treatment group and WHO steps (intent to treat).
(A) Subdomain scores; (B) summary scores.
SF-36: 36-item Short-Form Health Survey.
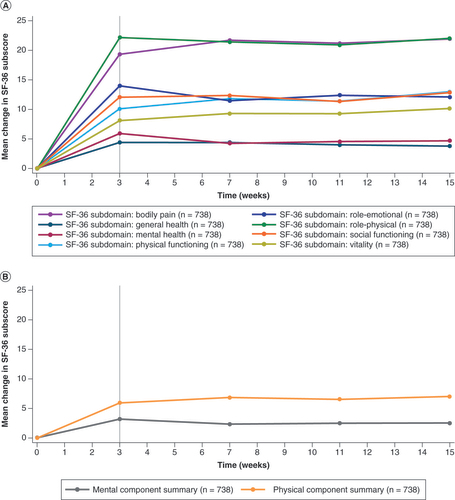
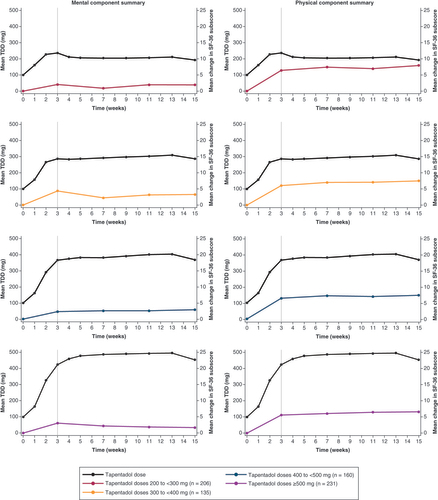
SF-36: 36-item Short-Form Health Survey; TDD: Total daily dose.
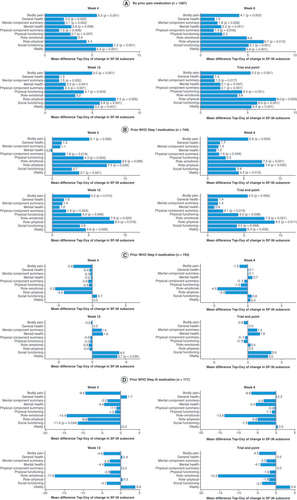
Least squares mean from ANCOVA of change from baseline in SF-36 score at the specified week with factors treatment group and pooled site, and covariate baseline score. Missing SF-36 subdomain scores imputed using last observation carried forward over the 15-week treatment period. p-values only shown where point estimate was significantly different from zero.
ANCOVA: Analysis of covariance; Oxy: Oxycodone; SF-36: 36-item Short-Form Health Survey; Tap: Tapentadol.
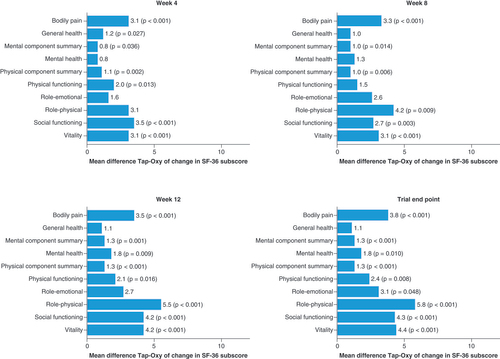
Least squares mean from ANCOVA for change from baseline in SF-36 score at the specified week with factors treatment group and pooled site and covariate baseline score. Missing SF-36 subdomain scores imputed using last observation carried forward over the 15-week treatment period. p-values only shown where point estimate was significantly different from zero.
ANCOVA: Analysis of covariance; Oxy: Oxycodone; SF-36: 36-item Short-Form Health Survey; Tap: Tapentadol.