Figures & data
Supplementary Table 1 presents a short description of the questionnaires used for collecting outcomes data.
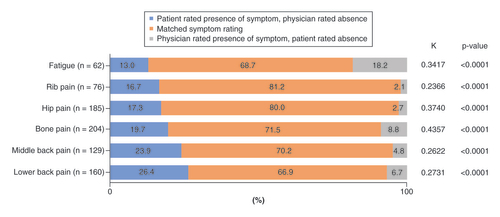
*Reported symptom concordance was based on data for 330 patient–physician pairs. Each physician could be matched with more than one patient.
Table 1. Patient demographic and clinical characteristics at face-to-face consultation.
Table 2. Proportions of patients experiencing clinically important symptom or functional impairmentTable Footnote†.
MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Physical pain (EORTC QLQ-MY20 Disease symptoms) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.
BTA: Bone-targeting agent; MM: Multiple myeloma.
![Figure 2. (A) Physical pain (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3 pain) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation.MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Physical pain (EORTC QLQ-MY20 Disease symptoms) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.BTA: Bone-targeting agent; MM: Multiple myeloma.](/cms/asset/a177517b-505b-4b52-bec6-9f3a365171ef/ipmt_a_12344458_f0002.jpg)
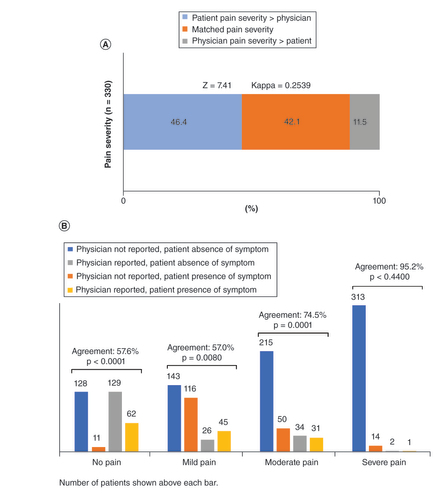
(B) Agreement between patient- and physician-reported pain severity by pain severity category. Number of patients shown above each bar.
MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; Bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Emotional pain (EORTC QLQ-MY20 Future perspective) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for one year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.
BTA: Bone-targeting agent; MM: Multiple myeloma.
![Figure 5. (A) Emotional pain (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3 Emotional functioning) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation.MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; Bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. (B) Emotional pain (EORTC QLQ-MY20 Future perspective) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation. MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for one year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation. No clinical threshold available for responses to the MY20 questionnaire.BTA: Bone-targeting agent; MM: Multiple myeloma.](/cms/asset/d71659f6-887f-416e-8378-f0b121171586/ipmt_a_12344458_f0005.jpg)
MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation.
BTA: Bone-targeting agent; MM: Multiple myeloma.
![Figure 6. Social pain (European Organisation for Research and Treatment of Cancer Quality of Life Core-30 Questionnaire version 3 Social functioning) directly communicated by patients via self-reports, as experienced in the past 7 days up to face-to-face clinical consultation.MM <1 year, patients diagnosed and living with MM for less than a year; MM ≥1 year, patients diagnosed and living with MM for 1 year or more; bone pain, patients with self-reported bone pain at the time of face-to-face consultation (data on patients with no self-reported bone pain at face-to-face consultation are not presented due to the small sample size [n = 41]); BTA, patients with a record of current bone-targeting agent (any) therapy at the time of face-to-face consultation; analgesic therapy, patients with a record of current analgesic therapy at the time of face-to-face consultation.BTA: Bone-targeting agent; MM: Multiple myeloma.](/cms/asset/a48492f0-9633-4d61-a96d-95e685db3960/ipmt_a_12344458_f0006.jpg)
Supplemental Document
Download MS Word (48 KB)Data sharing statement
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Real World Multiple Myeloma Disease Specific Programme. The analysis described in this study used data obtained from this survey and was funded by Amgen Ltd, who did not influence the original survey through either contribution to the design of questionnaires or data collection. All data that support the findings of this study are the intellectual property of Adelphi Real World and so are not publicly available. Data are however available upon reasonable request and with permission of Adelphi Real World.
