Figures & data
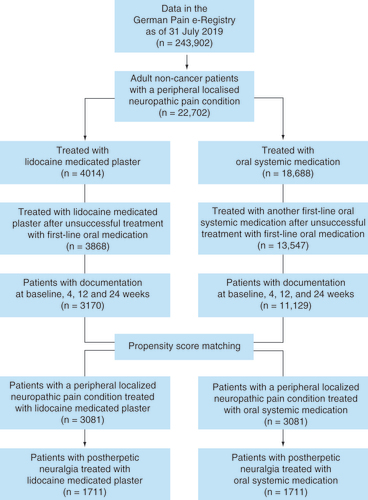
Table 1. Baseline characteristics before and after propensity score matching.
Table 2. Effectiveness assessment parameters at baseline and end of observation.
Table 3. Oral systemic recommended first-line medications documented for postherpetic neuralgia treatment in the oral systemic first-line medication group after switch from previous medication (n = 1711).
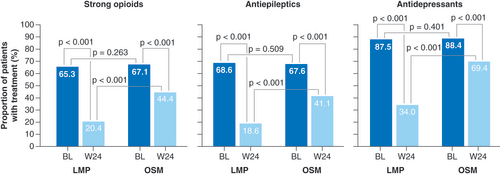
Strong opioids included morphine, hydromorphone, oxycodone ± naloxone, fentanyl, buprenorphine, tapentadol and others.
BL: Baseline; LMP: Lidocaine 700 mg medicated plaster; OSM: Oral systemic medication; W: Week.
(A) Change from baseline over the observation period. Boxplots show median (middle horizontal line in the box), 25 and 75% quartiles (bottom and top lines of the box), and five and 95% percentiles (whiskers). Improvement is shown by reductions in pain intensity index and modified pain disability index, and by increases in quality of life impairment by pain. (B) Improvement versus baseline in pain intensity index at end of observation.
LMP: Lidocaine 700 mg medicated plaster; NRS: Numerical rating scale; OSM: Oral systemic medication; PIX: Pain intensity index; VAS: Visual analogue scale.
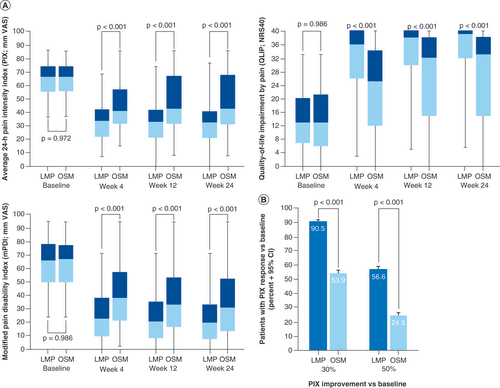
Table 4. Absolute change versus baseline in pain intensity index (primary effectiveness end point).
p < 0.001 in favor of lidocaine 700 mg medicated plaster.
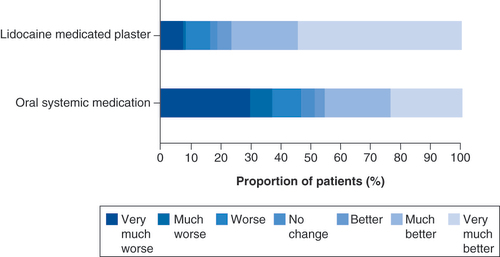
Boxplots show median (middle horizontal line in the box), 25 and 75% quartiles (bottom and top lines of the box), and five and 95% percentiles (whiskers).
BL: Baseline; MCS: Mental component score; PCS: Physical component score; W: Week.
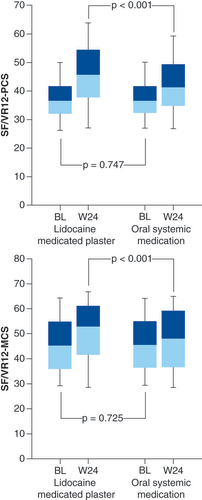
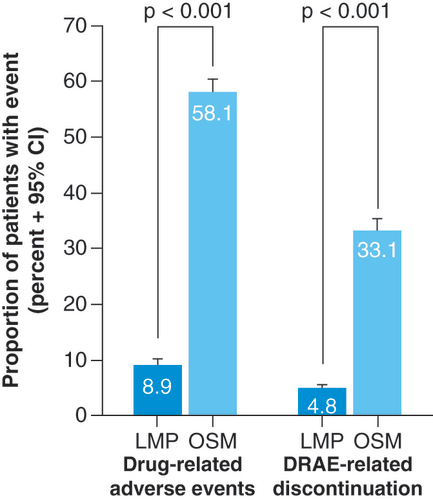
DRAE: Drug-related adverse event; LMP: Lidocaine 700 mg medicated plaster; OSM: Oral systemic medication.