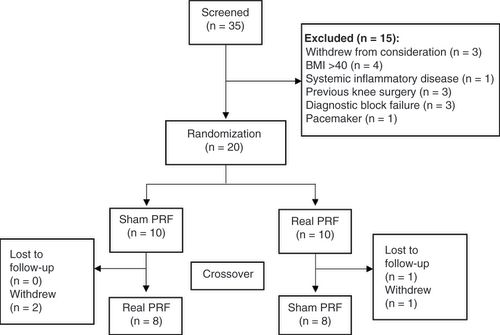
Figures & data
Table 1. Clinical and demographical characteristics at screening of 16 participants who completed both arms of the crossover intervention.
(A) Pain Intensity measured by NRS at study time points (baseline [T0] and after 2 weeks [T1], 1 month [T2], 3 months [T3] and 6 months [T4]). A statistically significant pain reduction was observed only after real PRF until 1-month after the procedure. (B) OKS at study time points (baseline [T0] and after 2 weeks [T1], 1 month [T2], 3 months [T3] and 6 months [T4]). A statistically significant improvement of function was observed only after real PRF until 3 months after the procedure.
m: Mean; NRS: Numerical rating scale; OKS: Oxford Knee Score; PRF: Pulsed radiofrequency; SD: Standard deviation.
![Figure 2. Within-groups variation of pain intensity and OKS at study time-points compared to baseline. (A) Pain Intensity measured by NRS at study time points (baseline [T0] and after 2 weeks [T1], 1 month [T2], 3 months [T3] and 6 months [T4]). A statistically significant pain reduction was observed only after real PRF until 1-month after the procedure. (B) OKS at study time points (baseline [T0] and after 2 weeks [T1], 1 month [T2], 3 months [T3] and 6 months [T4]). A statistically significant improvement of function was observed only after real PRF until 3 months after the procedure.m: Mean; NRS: Numerical rating scale; OKS: Oxford Knee Score; PRF: Pulsed radiofrequency; SD: Standard deviation.](/cms/asset/77daeeeb-9eaa-4158-bb55-bf26d068adea/ipmt_a_12344472_f0002.jpg)
Table 2. SF-36 domains at study time points (baseline [T0], after 2 weeks [T1], 1 month [T2], 3 months [T3] and 6 months [T4]).
Table 3. Between groups changes in primary and secondary outcomes (ANOVA with repeated measures).
Data sharing statement
The authors certify that this manuscript reports original clinical trial data. Deidentified, individual data that underlie the results reported in this article (text, tables, figures and appendices), along with the study protocol, will be available for meta-analyses for five years following publication. Parties seeking access to this information should submit a formal request to the corresponding author of this study.