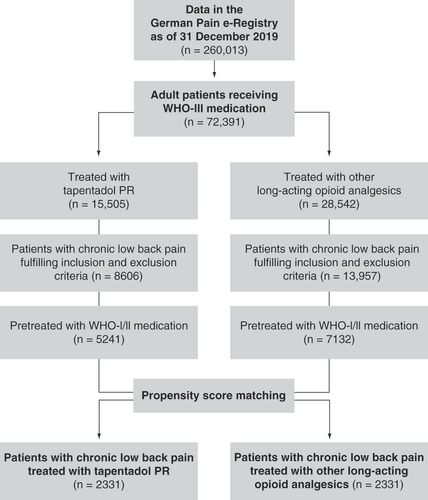
Figures & data
Table 1. Baseline characteristics (n = 2331 in each group).
Table 2. Previous and concomitant analgesic medications (n = 2331 in each group).
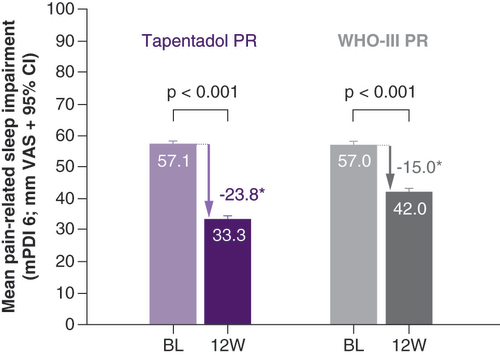
PR: Prolonged release.
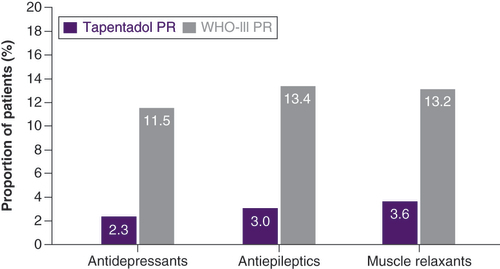
*p < 0.001 in favor of tapentadol PR.
BL: Baseline; mPDI: Modified pain disability index; PR: Prolonged release; SF: Short form; VAS: Visual analogue scale; W: Week.
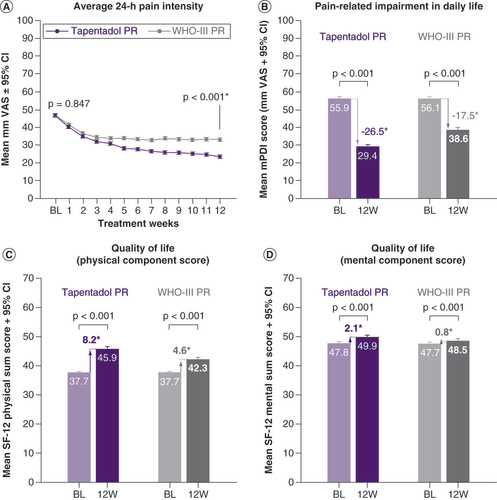
Table 3. Mean changes in pain intensity over the evaluation period (n = 2331 in each group).
*p < 0.001 in favor of tapentadol PR.
mPDI: Modified pain disability index; PR: Prolonged release; VAS: Visual analogue scale; W: Week.
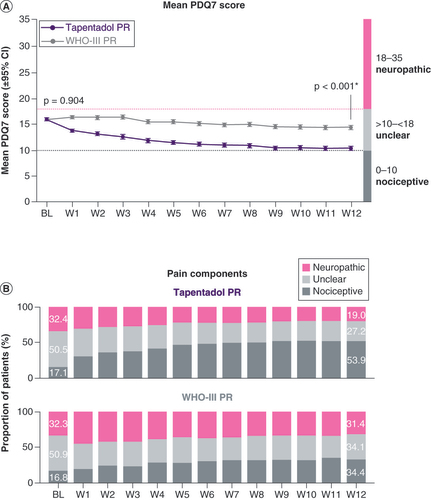
(A) Mean PDQ7 score. (B) Pain components.
*p < 0.001 in favor of tapentadol PR.
BL: Baseline; PDQ: PainDETECT questionnaire; PR: Prolonged release; W: Week.
A BFI reference range of 0–28.8 indicates normal bowel function.
BFI: Bowel function index; BL: Baseline; PR: Prolonged release; VAS: Visual analogue scale; W: Week.
Reproduced with permission from the International Association for the Study of Pain [Citation40].
![Figure 6. Change in bowel function over the observation period.A BFI reference range of 0–28.8 indicates normal bowel function.BFI: Bowel function index; BL: Baseline; PR: Prolonged release; VAS: Visual analogue scale; W: Week.Reproduced with permission from the International Association for the Study of Pain [Citation40].](/cms/asset/603c1ecc-48fc-41f0-bc80-27a8c0d96ecd/ipmt_a_12344475_f0006.jpg)
Table 4. Drug-related adverse events during the 12-week observation period (n = 2331 in each group).
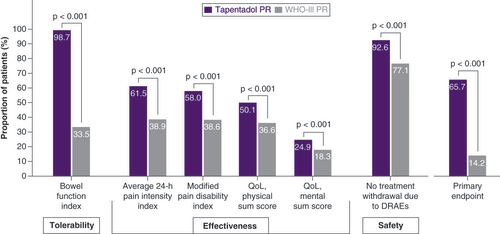
The primary end point included the three dimensions: tolerability, effectiveness and safety. A treatment responder had to fulfil the tolerability criterion and the safety criterion plus at least two criteria from the dimension effectiveness.
DRAE: Drug-related adverse event; PR: Prolonged release; QoL: Quality of life.