Figures & data
Table 1. Baseline characteristics of all patients with localized neuropathic pain conditions (n = 3081 in each group).
Table 2. Baseline characteristics of all patients with localized neuropathic pain conditions treated with the lidocaine 700 mg medicated plaster stratified for patients with postherpetic neuralgia (PHN) and patients with diabetic polyneuropathy, postsurgical neuropathy or other peripheral localized neuropathic pain conditions (l-PNP other than PHN).
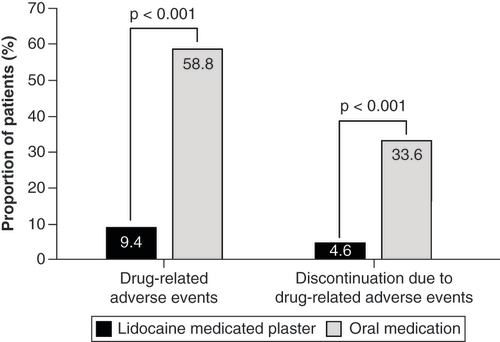
Table 3. Main reasons for discontinuation from treatment.
Table 4. Oral recommended first-line medications documented for the treatment of localized neuropathic pain conditions in the OM group after switching from previous medication (n = 3081).
Table 5. Change in concomitant analgesic medication at end of observation.
Table 6. Absolute mean change from baseline (standard error) in average 24-h pain intensity index (averaged over 4, 12 and 24 weeks after treatment initiation).
Table 7. Absolute mean change from baseline (standard deviation) in average 24-h pain intensity index over the observation period in the overall localized peripheral neuropathic pain population.
Table 8. Proportion of patients in the overall localized peripheral neuropathic pain population with a treatment response (≥30% and ≥50% reduction in the average 24-h pain intensity index) over the observation period.
Table 9. Mean change from baseline (standard error) in further effectiveness parameters in the overall localized peripheral neuropathic pain population (averaged over 4, 12 and 24 weeks after treatment initiation).
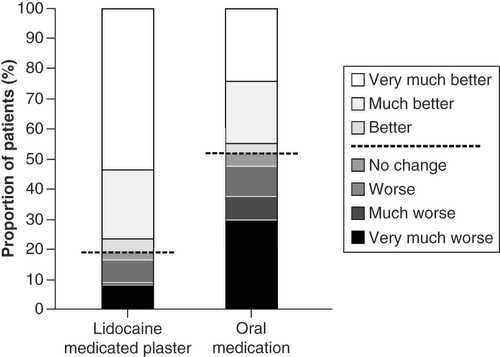
All patients above the dashed line reported improvement. p < 0.001 in favor of lidocaine 700 mg medicated plaster.