Figures & data
Table 1. Baseline characteristics of all patients with postsurgical neuropathic pain.
OM: Oral first-line medication; SSNRI: Selective serotonin-norepinephrine reuptake inhibitor.
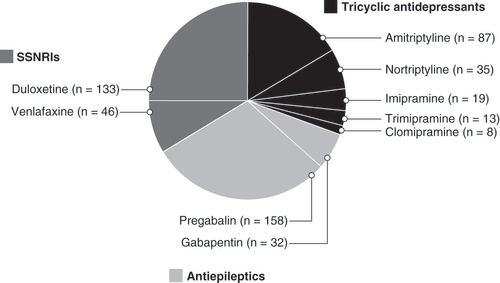
Data for nonopioids and mild opioids are not shown.
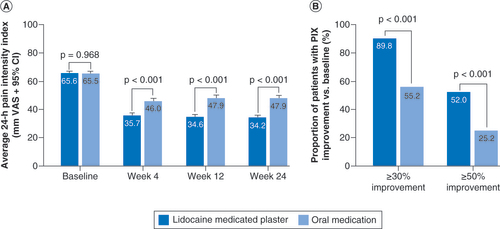
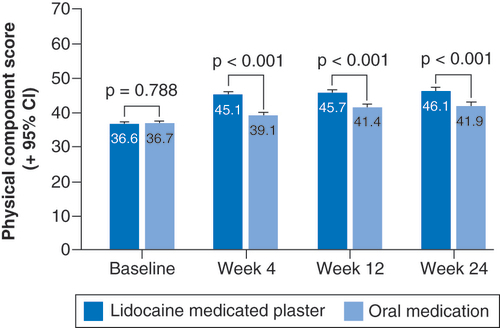
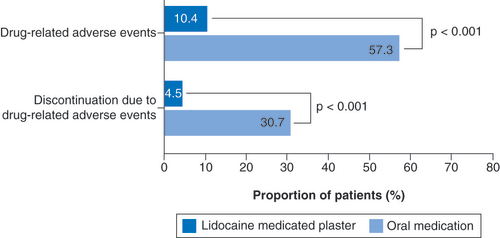
LMP: Lidocaine 700 mg medicated plaster; OM: Oral medication.
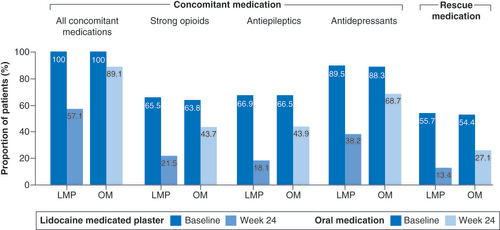
(A) Change from baseline over the observation period. (B) Improvement versus baseline at end of observation.
PIX: Pain intensity index.