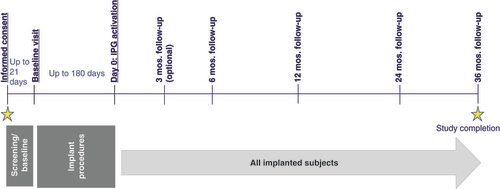
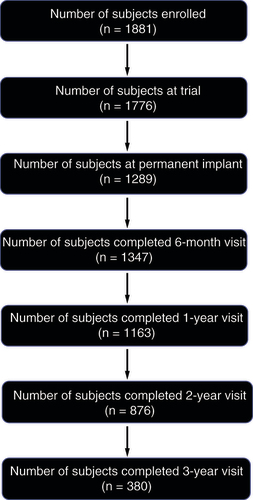
Figures & data
Table 1. Demographics for implantedTable Footnote† subjects.
Table 2. Device-related complications including explants up to 3 years (n = 1289 implanted participants).
Table 3. Serious adverse events (related to either device, procedure or stimulation), totaling 32 serious adverse events related to device and 51 events related to procedure (overlapping events may be reported) reported among the 1881 consenting patients.
Data sharing statement
The data, analytic methods and study materials for this clinical study will be made available to other researchers in accordance with Boston Scientific Data Sharing Policy (https://www.bostonscientific.com).