Figures & data
Based on 2022 guidelines on the treatment of PDPN from the American Association of Clinical Endocrinology [Citation27] and Clinical Compendia American Diabetes Association [Citation28].
*Indicates FDA-approved for PDPN.
PDPN: Painful diabetic peripheral neuropathy.
![Figure 1. Place of capsaicin 8% topical systems in the pharmacological treatment of painful diabetic peripheral neuropathy of the feet.Based on 2022 guidelines on the treatment of PDPN from the American Association of Clinical Endocrinology [Citation27] and Clinical Compendia American Diabetes Association [Citation28].*Indicates FDA-approved for PDPN.PDPN: Painful diabetic peripheral neuropathy.](/cms/asset/09ab4e06-5777-4239-9dfd-7d2e0ce26e56/ipmt_a_12344556_f0001.jpg)
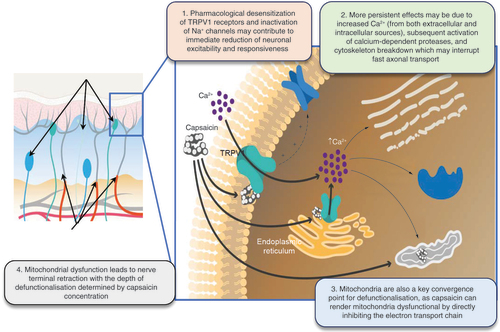
Left panel: Upper black arrows indicate capsaicin-insensitive nerve terminals. Lower black arrows indicate nerve terminal retraction due to mitochondrial dysfunction.
The topical system consists of a polyester-film backing layer coated with a matrix composed of capsaicin, a DGME-containing solvent, silicone adhesive mixtures, and other ingredients, and covered with a removable polyester-release liner. As DGME is extremely lipophilic, it is easily absorbed into the epidermal and dermal layers but has little affinity for the blood phase. During application, capsaicin is diffused into the skin via the influx of DGME and efflux of water, forming a reservoir of bioavailable capsaicin [Citation20,Citation45].
DGME: Diethylene glycol monomethyl ether.
![Figure 3. Technology of the capsaicin 8% topical system, showing the transfer of capsaicin into the skin during the application period, enabling long-term (3-month) pain relief.The topical system consists of a polyester-film backing layer coated with a matrix composed of capsaicin, a DGME-containing solvent, silicone adhesive mixtures, and other ingredients, and covered with a removable polyester-release liner. As DGME is extremely lipophilic, it is easily absorbed into the epidermal and dermal layers but has little affinity for the blood phase. During application, capsaicin is diffused into the skin via the influx of DGME and efflux of water, forming a reservoir of bioavailable capsaicin [Citation20,Citation45].DGME: Diethylene glycol monomethyl ether.](/cms/asset/ab2e8526-5252-4f5a-91b5-25defe9611fe/ipmt_a_12344556_f0003.jpg)
Details are based on the US prescribing information [Citation20].
![Figure 4. Summary of the instructions for applying the capsaicin 8% topical system.Details are based on the US prescribing information [Citation20].](/cms/asset/8636641e-d16c-4fec-8ff0-1a375212d51e/ipmt_a_12344556_f0004.jpg)

