Figures & data
*Dosing occurred after 10 h fast.
Table 1. Participant characteristics.
(A) Linear scale, (B) semilogarithmic plot. Error bars represent standard deviation.
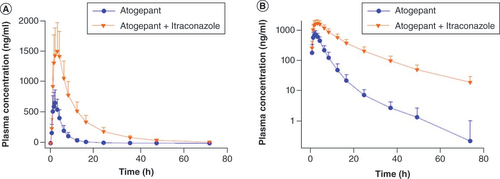
Table 2. Pharmacokinetic parameters of atogepant following administration with and without itraconazole in healthy participants; Study A (N = 40).
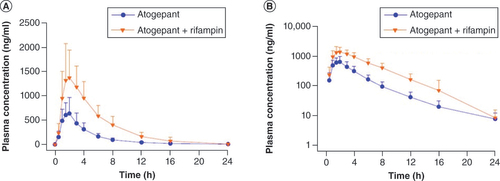
(A) Linear scale, (B) semilogarithmic plot. Error bars represent standard deviation.
(A) Linear scale, (B) semilogarithmic plot. Error bars represent standard deviation.
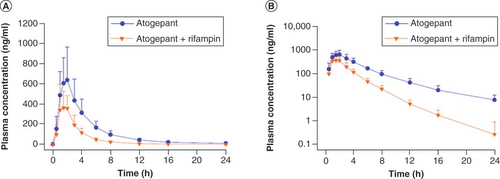
Table 3. Pharmacokinetic parameters of atogepant following administration with and without rifampin in healthy participants; study B (N = 31).
Table 4. Number (%) of participants with treatment-emergent adverse events (TEAE) by study treatment, system organ class and preferred term (safety population).
Data sharing statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/
