Figures & data
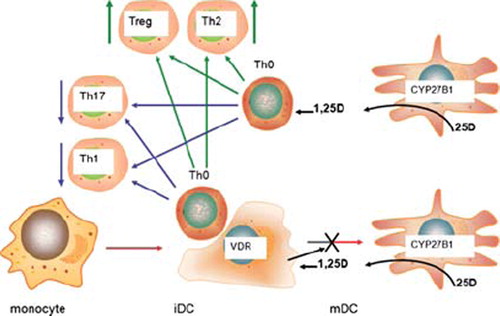
Figure 1. Intracrine metabolism of vitamin D and monocyte antibacterial immunity. Bacterial pathogens are phagocytosed by cells such as monocytes but are the able to undergo intracellular replication that may threaten the host cell. Pattern recognition receptors such as the toll-like receptor (TLR) family can also act as monocyte sensors of pathogens, with concomitant effects on expression of genes such as CYP27B1 and VDR. Induction of these components of the vitamin D system provides an mechanism for conversion of 25(OH)D (25D) to 1,25(OH)2D (1,25D) and subsequent intracrine nuclear signaling via the VDR. Amongst the genes induced by the intracrine system is cathelicidin (LL37) which acts as an antibacterial protein when incorporated into the phagosome. Other antibacterial factors such as β-defensin 2 (DEFB4) are also induced by intracrine 1,25D, but require additional stimulation by nuclear factor-kappa B (NF-κB), stimulated by factors such as interleukin-1 (IL-1) or NOD2 and its ligand muramyl dipeptide (MDP). Intracrine metabolism of vitamin D also promotes bacterial killing via enhanced autophagy which promotes formation of an autophagosome. Induction of an intracrine vitamin D system in monocytes via TLR activation appears to require interleukin-15 (IL-15) as an intermediary and can also be stimulated by interferon γ (IFNγ), with enhanced antibacterial activity. Conversely, interleukin-4 (IL-4) suppresses intracrine antibacterial activity of vitamin D by enhancing the vitamin D catabolic enzyme 24-hydroxylase (CYP24A1).
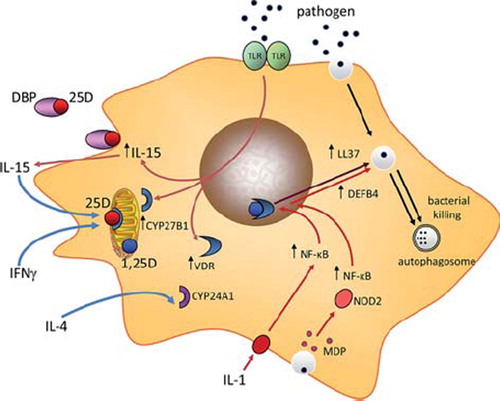
Figure 2. Leprosy, a model for disease-associated dysregulation of intracrine vitamin D. The disease leprosy is associated with infection by Mycobacterium leprae or Mycobacterium lepromatosis (mLep), with mLep signaling via TLR2/1. Two forms of leprosy, Tuberculoid leprosy (T-lep) and Lepromatous leprosy (M-lep) are associated with distinct cytokine profiles: IFNγ and IL-15 in T-lep and interferon α (IFNα), IL-4 and interleukin-10 (IL-10) in L-lep. L-lep monocytes also express the microRNA, miR-21 that targets expression of the CYP27B1 gene product. DNA array analysis of T-lep and L-lep lesions, as well as reversal reaction (rr) L-lep lesion reveals differential patterns of gene expression for CYP27B1, VDR and CYP24A1, with expression of all three gene being decreased in L-lep. Expression of CYP27B1, VDR and CYP24A1 is retsored in L-lep lessions that undergo spontaneous conversion to T-lep (rr). The enhanced intracrine vitamin D system in T-lep monocytes enables synthesis of 1,25(OH)2D (1,25D) from 25(OH)D (25D) and associated antimycobacterial activity in these cells. By contrast, in L-lep IL-10 promotes phagocytosis but without an effective intracrine vitamin D system to kill phagocytosed mycobacteria.
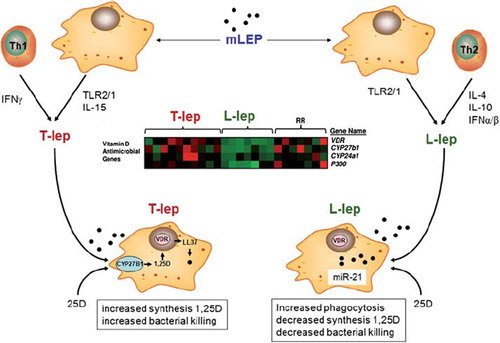
Figure 3. Paracrine metabolism of 25(OH)D and the regulation of adaptive immunity. Synthesis of 1,25(OH)2D (1,25D) from 25(OH)D (25D) in mature dendritic cells (mDC) expressing CYP27B1 can exert effects on: 1) immature DCs (iDC) expressing VDR; 2) activated T cells (Th0) expressing VDR. Resulting effects on Th0 cells include: 1) activation of regulatory T cells (Treg); 2) activation of Th2 cells; 3) suppression of interleukin-17 (IL-17) expressing Th17 cells; suppression of Th1 cells.