Figures & data
Table I. Sensitivity, specificity, positive and negative predictive values of various markers on the likelihood of achieving SVR in patients with HCV genotype 2 (n = 50) and genotype 3 (n = 111) included in the per-protocol analysis treated for 12 weeks.
Figure 1. Proportion of HCV genotype 2 (A) or 3 (B) infected patients achieving SVR following 12 or 24 weeks included in the intention-to-treat (ITT) and per-protocol (PP) analyses among patients achieving <1000 vs. ≥1000 IU/mL day 7 in the NORDynamIC trial. The number of patients under each column reflects the ITT population.
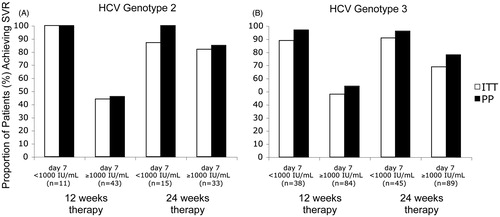
Figure 2. Proportion of HCV genotype 2 (A) or 3 (B) infected patients achieving SVR following 12 or 24 weeks included in the intention-to-treat (ITT) and per-protocol (PP) analyses among patients with age <40 vs. ≥40 years in the NORDynamIC trial. The number of patients under each column reflects the ITT population.
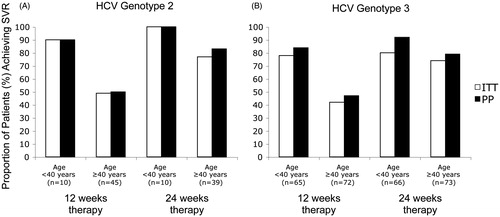
Table II. Real-Life SVR data among Finish HCV genotype 2/3 infected patients where duration is determined by age; Ribavirin 800 mg per day.
Table III. Real-life SVR data among Swedish genotype 2/3 infected patients achieving <1000 IU/mL day 7 or age <40 years treated for 12–16 weeks.
