Figures & data
Table 1. Behaviour of HSF−/− and HSF1+/+ in the elevated plus maze.
Figure 1. Behavioural analysis in open field test. Mean number of squares crossed in 5 min. Significant anxiolytic action of hyperthermia was seen in hsf1+/+ mice, but not in hsf1−/− mice. NS group: non-experimental stress group; HS group: hyperthermia group; CUS group: chronic unpredictable stress group; HS + CUS group: hyperthermia + chronic unpredictable stress group. *p < 0.05, #p < 0.05 versus NS and HS groups; **p < 0.05, ##p < 0.05 versus CUS group; OF: open field, N = 14.
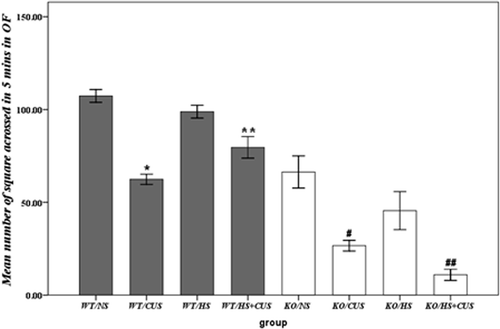
Figure 2. Western blot analysis of Hsp72 expression in mice hippocampus. Upper panel: semi-quantitative analysis of Hsp72 expression. In hsf1+/+ mice, mice in HS, and HS + CUS groups were treated with hyperthermia (39° ± 2°C) for 15 min. The Hsp72 expression was significantly higher in HS and HS + CUS groups than NS and CUS groups after 8 weeks (*p < 0.05). In hsf1−/− mice, Hsp72 expression was abolished in all groups. Lower panel: representative Western blot of HSP and β-actin expression. Animals were grouped as described in .
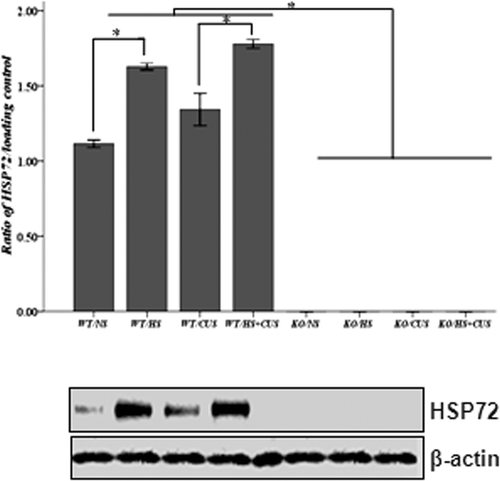
Figure 3. Apoptosis in hippocampal CA3 area in the hsf1+/+ mice. (A) TUNEL staining of hippocampal CA3 area, original magnification ×400. The apoptotic cells exhibited condensed chromatin and smaller, dark brown nucleus. (B) The amount of apoptotic cells in WT mice. In hsf1+/+ mice, chronic unpredictable stress increase apoptosis in the CA3, but hyperthermia decrease chronic unpredictable stress induced apoptosis (n = 8–12). *p < 0.05 versus NS. Animals were grouped as described in .
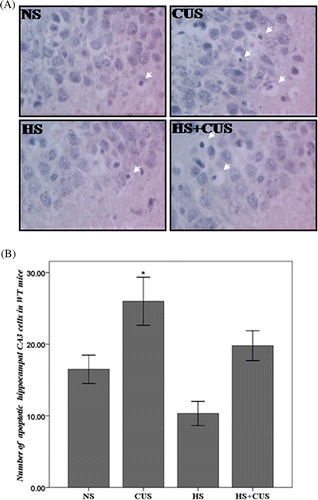
Figure 4. Apoptosis in hippocampal CA3 area in the hsf1−/− mice. (A) TUNEL staining of hippocampal CA3 area, original magnification ×400. The apoptotic cells exhibited condensed chromatin and smaller, dark brown nucleus. (B) The amount of apoptotic cell in hsf1−/− mice. Chronic unpredictable stress increased apoptosis in the CA3 cells, but hyperthermia further increase CUS-induced apoptosis (n = 8–12). *p < 0.01 versus NS. Animals were grouped as described in .
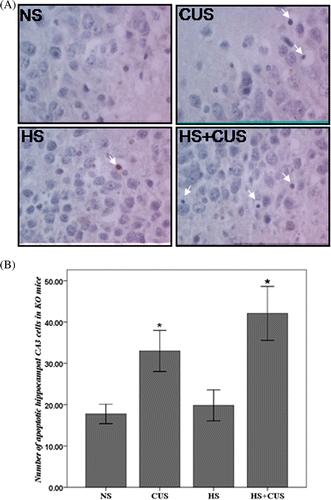
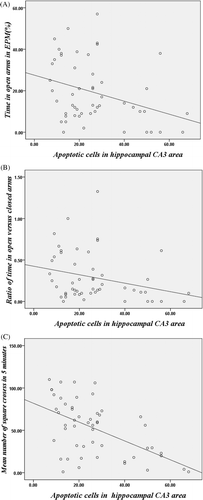
Figure 5. Correlation between apoptotic hippocampal CA3 cells and anxiety-like behaviours. (A) Correlation between the number of apoptotic hippocampal CA3 cells and the percentage of time spent in the open arm in EPM test (r = −0.33, p < 0.05). (B) Correlation between the number of apoptotic hippocampal CA3 cells and the ratio of time in open versus closed arms in EPM test (r = −0.28, p < 0.05). (C) Correlation between the number of apoptotic hippocampal CA3 cells and the mean number of square crossed in 5 min in the open field test (r = −0.54, p < 0.05).