Figures & data
Figure 1. MRTI Imaging of AuNS Mediated Heating Schematic - Tumors were grown on the backs of SCID mice. 24-hours prior to treatment, PEGylated AuNS suspended in phosphate buffered saline were injected via tail vein and allowed to circulate. On treatment day, mice were anesthetized and tumors illuminated with a laser under real-time magnetic resonance temperature image monitoring.
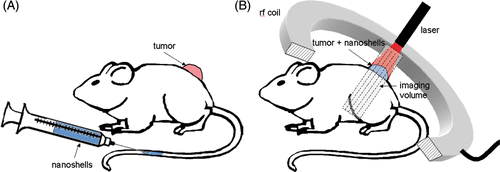
Figure 2. AuNS Mediated Heating – Calculated maximum change in temperature (color) is mapped onto T2-W anatomical images of the mouse xenografts (column a). Relatively low heating is observed in the control (top) versus the highly tumor specific heating observed in the +AuNS tumor (bottom). The cumulative minutes at 43°C (t43) thermal dose was estimated from the temperature history (column b) and the 240 min (red) and 90 min (yellow) isotherms are shown to demonstrate the rapid dose gradient with depth in tissue. H&E staining of tissue post-treatment (column c) demonstrates the correlation between observed damage (red) in the +AuNS versus the expectations from MRTI (columns a&b).
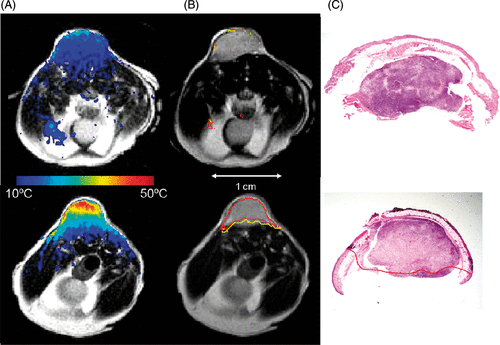
Figure 3. MRTI Evaluation of Spatiotemporal Temperature Changes – Gaussian distributions were fit (solid lines) to binned histogram data of temperature changes in a control (○) and +AuNS (Δ) tumors (a) to further demonstrate the clear differences (19.2°C) in temperature distribution observed between a control (ΔTmax = 17.6°C) and +AuNS (ΔTmax = 36.8°C) tumors. Temporal temperature history (b) demonstrated the rapid heating of +AuNS (X) versus a control (·) over the 180 sec exposure at 4 W/cm2 and the shape of the curve at maximum temperature correlated well with theory (solid lines). Spatial profiles of the temperature (c) taken at 45 sec (▴), 90 sec (•) and 180 sec (▪) for a +AuNS tumor help demonstrate that the primary source of heating came from just distal to the skin surface within the cortex of the tumor when correlated with the region of tumor on T2-W MRI (—-).
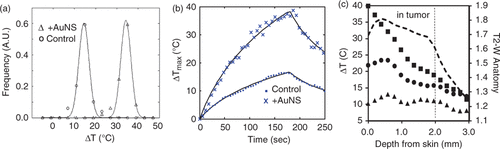
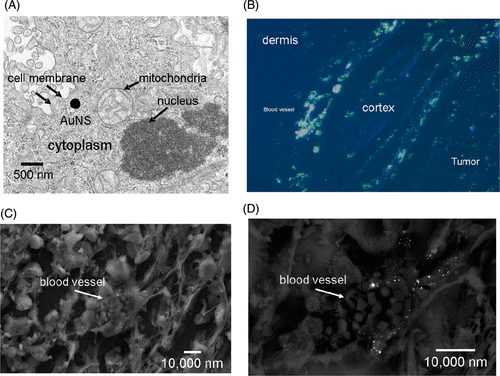
Figure 4. Microscopy of AuNS in PC-3 xenografts – TEM illustrates the size of the AuNS (140–150 nm) in relation to cellular organelles (a). Silver staining of the AuNS on polarized fluorescent microscopy (b) corroborated the observation that uptake near blood vessels which were most plentiful in the peripheral tumor cortex. SEM of individual vessels (c) further implied that the passively extravasated AuNS tended to cluster in the perivascular space (d).