Figures & data
Figure 1. Immunocytochemistry for Hsp27 (HSPB1) and Hsp72 (HSPA1A) proteins in hMLH1-deficient (HCT116) and hMLH1-proficient (HCT116 + ch3) cell lines. Panel I. Images of immunostained cells. NC, negative control (non-immune IgG control). Control group (37°C) and cells exposed to heat treatment at 41°C or 42°C for 1 h. Images were taken with a 40 × objective. Panel II. Immunostaining quantification. (A, C) Percentage of cells with positive immunostaining for cytoplasmic HSPB1and HSPA1A, respectively. (B, D) Percentage of cells with nuclear positive immunostaining for HSPB1 and HSPA1A, respectively. C = control at 37°C. Cells were exposed to heat treatment at 41° or 42°C for 1 h, and then incubated at 37°C at the indicated times (0 = immediately after heat shock). Points represent mean ± SEM calculated from three independent experiments. Comparison with the control group (#) and between cell lines (*),#*P < 0.05, ##**P < 0.01, ###***P < 0.001.
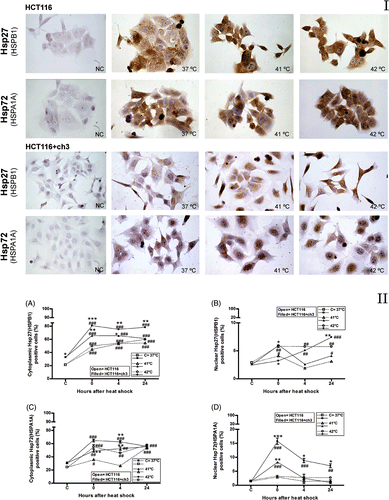
Figure 2. Immunoblots of Hsp27 (HSPB1), Hsp72 (HSPA1A), hMLH1 and hMSH2 proteins in the cytoplasmic and nuclear fractions of HCT116 and HCT116 + ch3 cells exposed to heat treatment. Panel I. Cells were heated at 41° or 42°C for 1 h, and then the cytoplasmic and nuclear protein fractions were extracted and analysed by western blot: immediately after hyperthermia (0), 4 and 24 h after heating. Control unheated cells were also included (37°C). HS: heat shock. As loading standard for cytoplasmic proteins we used α-tubulin and for nuclear proteins we used HDAC1. Data are representative of three independent experiments. Panel II. (A, C) Ratio of band intensity for cytoplasmic HSPB1and HSPA1A, respectively. (B, D) Ratio of band intensity of nuclear HSPB1 and HSPA1A, respectively. The ratio of band intensity to three independent experiments, as shown in Panel I, was quantified by densitometry as described in the text. The means ± SEM are plotted. Statistical differences were calculated with respect to the unheated control group (37°C,#) and between hMLH1-proficient and hMLH1-deficient cells (*). #*P < 0.05, ##**P < 0.01, ###***P < 0.001.
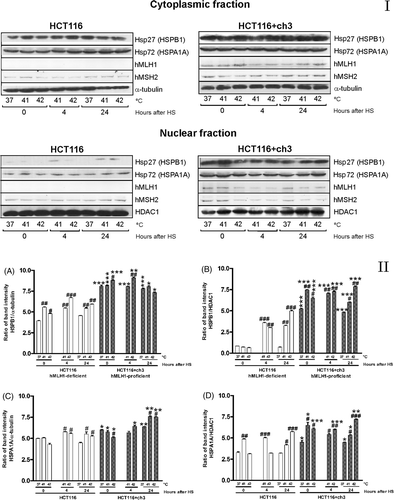
Figure 3. De-localisation of hMLH1 and hMSH2 under heat shock in HCT116 and HCT116 + ch3 cells. (A) hMLH1 protein expression in cytoplasmic and nuclear compartment in HCT116 + ch3 tumour cells. (B, C) hMSH2 protein expression in cytoplasmic and nuclear compartment in HCT116 + ch3 and HCT116 tumour cells, respectively. Cells were exposed to a heat treatment at 41° or 42°C for 1 h, and then the cytoplasmic and nuclear protein fractions were analysed by western blot immediately after hyperthermia (0), 4 and 24 h post-treatment. HS: heat shock. The ratio of band intensity corresponds to three independent experiments as shown in . The means ± SEM are plotted. Statistical differences were calculated with respect to the unheated control group (C = 37°C,#) and between nuclear and cytoplasmic fractions (*). #*P < 0.05, ##**P < 0.01, ###***P < 0.001.
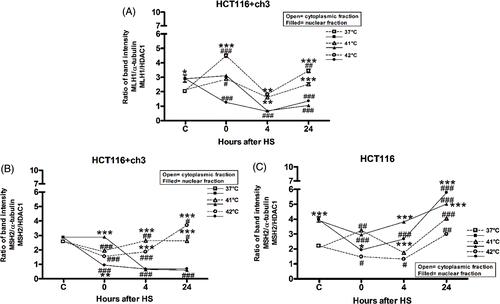
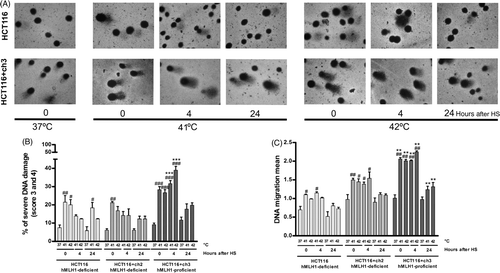
Figure 4. Effects of loss of MMR on the DNA damage induced by hyperthermia. MMR-deficient cells (HCT116 and HCT116 + ch2) and MMR-proficient cells (HCT116 + ch3) were exposed 1 h to 41° or 42°C or then collected to study the DNA damage by the comet assay immediately after hyperthermia (0), 4 and 24 h post-treatment. Control unheated cells were included (37°C). (A) Images of comets taken with 40 × objective. Note the increment of the comet tail length as a result of the DNA damage induced by the heat treatment in MMR-proficient cells (HCT116 + ch3). (B) DNA migration mean. (C) Percentage of cells with severe DNA damage (score 3: 31–60% and score 4: 61–95%). HS: heat shock. Statistical differences were calculated with respect to the unheated control group (#) and between hMLH1-proficient and hMLH1-deficient cells (*). #*P < 0.05, ##**P < 0.01, ###***P < 0.001. Data are representative of at least three independent experiments.