Figures & data
Figure 1. In vitro set up for sonication experiments. (A) A dish containing the sample (S) is positioned directly on top of the transducer (T) after applying acoustic gel to avoid air between the transducer and the dish. (B) Ultrasound travels horizontally through degassed distilled water to irradiate the sample positioned at a certain distance. At the far end is an ultrasound absorber (UA) that prevents reflection of ultrasound. (C) The tube containing the sample is positioned a few centimeters from the transducer and could be rotated during sonication for a more uniform exposure of its content. Ultrasound is directed upwards to hit the sample. (D) For small samples (such as those using 24 or 96 well plates), small transducers (e.g. less than 10 mm in diameter) can be dipped directly into the sample for sonication.
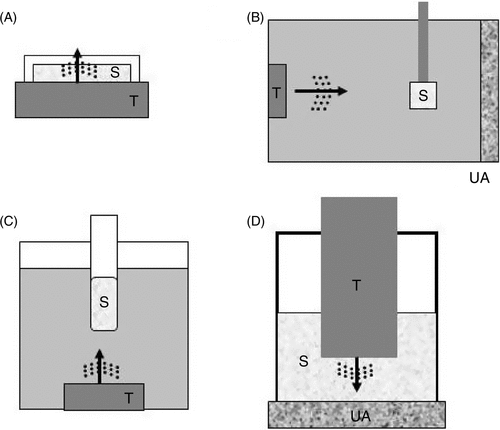
Table 1. Advantages and disadvantages among experimental systems
Figure 2. In vivo-simulated set up. This is a novel in vitro set-up that simulates in vivo conditions. Close chamber containing cancer cells is sandwiched in between transmitting oil simulating soft tissue in the body and ultrasound absorber on the opposite side to minimize reflection of ultrasound waves.
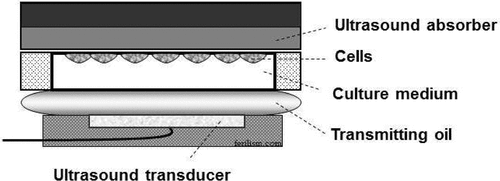
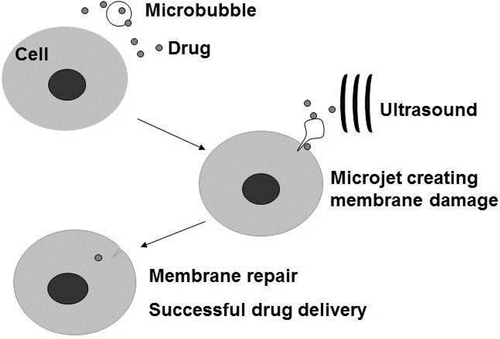
Figure 3. Targeted drug delivery by ultrasound with microbubbles. Microbubbles can be engineered to carry therapeutic agents and at the same time carry with them ligands that specifically latches on to particular cells (e.g. cancer cells belonging to a cancer cell line). Conceptually, these engineered microbubbles will be injected intravenously into a cancer patient, allowing time for the microbubble to localize on the cancer cells before sonication. Sonication will release and deliver the therapeutic agent into the target cells. Ultrasound in this case should reach an acoustic pressure sufficient to destroy the bubbles, but may generate mechanical and thermal effects in the surrounding tissue without or with fewer microbubbles and less drug. The expected effect would be greater on the target cells and tissues, with minimal side effects.