Figures & data
Figure 1. Hyperthermia modulates innate and adaptive immune responses. A general scheme of the main cells of the innate and adaptive immune systems that are involved in anti-tumour responses and can be modulated by hyperthermia is displayed. Heat damaged tumour cells expose damage-associated molecular patterns (DAMP) and can be recognised by innate immune cells via pattern recognition receptors (PRR). Natural killer (NK) cells are activated against the tumour via triggering activating receptors (AR) and inhibiting inhibitory receptors (IR) via MHC loss of the tumour cells. A beneficial cytokine milieu is created in the tumour microenvironment when innate immune cells are exposed to heat. Dendritic cells (DC) link the innate and adaptive immune response and take up heat shock protein/tumour antigen (Ag) complexes by way of heat shock protein receptors (HSPR). DCs present the tumour Ag to T cells and heat induces maturation and migration of DCs to lymph nodes (LN). There, T cells are activated in a MHC-dependent manner. They expand and traffick to the tumour cells by passing through high endothelial venules (HEV). Finally, the tumour cells are attacked and killed by the activated CD8+ T cells (cytotoxic T lymphocytes, CTL). As outlined in detail in the text, hyperthermia fosters in vivo all of the presented immune mechanisms against the tumour cells.
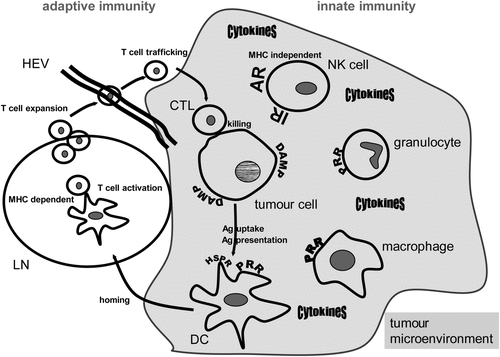
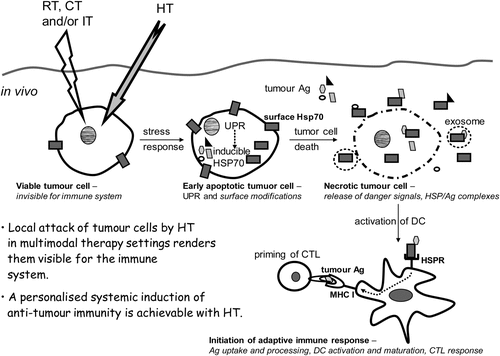
Figure 2. Hyperthermia modified tumour cells are rendered immunogenic and should be regarded as in situ tumour vaccine. When a tumour cell is heated, protein aggregation and denaturation induces a stress response in the cell, the so called unfolded protein response (UPR). Consequently, the transcription of inducible heat shock protein 70 (Hsp70) is increased and tumour cells expose even more Hsp70 on their surface. Furthermore, hyperthermia (HT) results in enhanced levels of tumour antigens (Ag) inside the cell. A second stress stimulus for the tumour cells such as ionising irradiation (radiotherapy, RT), chemotherapy (CT) or immune therapy (IT) together with HT fosters the induction of necrotic tumour cell death forms and modifies the tumour cell surface. Since necrotic cells have lost their membrane integrity, HSPs acting as danger signals and HSP/tumour Ag complexes are released. In addition, HSPs and tumour Ag containing exosomes can be discharged from apoptotic and necrotic tumour cells. Hsp70 containing exosomes derived from heat stressed tumour cells as well as HSP/tumour Ag complexes activate and attract dendritic cells (DC). The latter take up tumour Ag, present it with co-stimulation to CD8+ T cells and thereby induce cellular anti-tumour immunity by priming cytotoxic T lymphocytes (CTL).