Figures & data
Table I. Summary of the mild hyperthermia sonications performed in vivo and in vitro. Duration of 10 min and a frequency of 1.2 MHz were used for all sonications in vivo. Duration of 30 min and a frequency of 1.2 MHz were used for sonications in vitro. The animal used in the LTSL experiment is identified with an asterisk (*).
Figure 1. Schematic of the experimental MR-HIFU hyperthermia set-up, modified from Ranjan et al. Citation[66]. The sagittal imaging plane is shown, with the rabbit in right lateral decubitus position on top of the HIFU platform and the tumour-bearing right hind limb submerged in degassed water. Baseline reference temperature was obtained using a fibre-optic temperature probe inserted in the thigh muscle near the tumour. The imaging slice positions for the thermometry sequence are outlined with a blue dashed line, and the target region within the tumour is shown as a green circle. Depiction of transducer and HIFU beam propagation are meant to be illustrative.
![Figure 1. Schematic of the experimental MR-HIFU hyperthermia set-up, modified from Ranjan et al. Citation[66]. The sagittal imaging plane is shown, with the rabbit in right lateral decubitus position on top of the HIFU platform and the tumour-bearing right hind limb submerged in degassed water. Baseline reference temperature was obtained using a fibre-optic temperature probe inserted in the thigh muscle near the tumour. The imaging slice positions for the thermometry sequence are outlined with a blue dashed line, and the target region within the tumour is shown as a green circle. Depiction of transducer and HIFU beam propagation are meant to be illustrative.](/cms/asset/4dcf307a-13cb-49a9-bafd-2b4d5e4f4ae1/ihyt_a_680173_f0001_b.gif)
Figure 2. Planning and temperature mapping for mild hyperthermia: (A) VX2 tumour (hyper-intense) was clearly identified (white dashed line) on the proton density-weighted planning images and a target region within the tumour was chosen (green circle). (B) Temperature maps (colour scale) overlaid on planning images (greyscale) during a mild hyperthermia treatment with an 8 mm treatment cell, showing typical temperature distribution after 5 min of heating. Temperature monitoring and control was achieved in the selected target region with an FFE-EPI imaging sequence, utilising the PRFS method for temperature mapping, and by using the mild hyperthermia feedback control algorithm. The ROI used for magnetic drift correction is outlined with a white dashed line. C and D are the sagittal image planes corresponding to A and B, respectively.
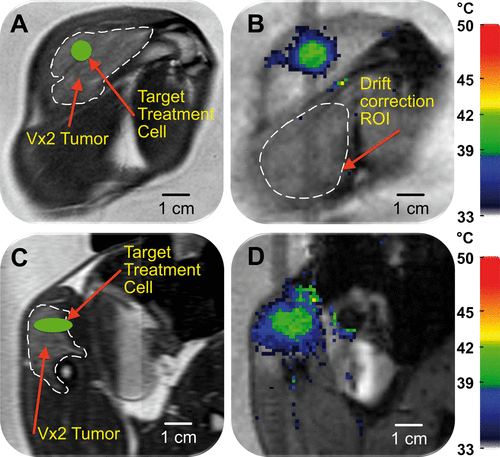
Figure 3. Mild hyperthermia feedback schematic. (A) During heat-up, sonication cycles through all heat-up trajectories. Once the criteria for every heat-up trajectory are met, heating is paused. When temperature in one of the monitored subtrajectories drops below the lower limit, sonication resumes on that subtrajectory until the upper limit is reached. The cycle of ‘wait’ and ‘maintain’ subtrajectories is repeated until the end of treatment. (B) The schematic demonstrates the flexibility of the binary feedback control algorithm. The algorithm sequentially heats from the innermost to the outermost subtrajectory during heat-up. After the outermost subtrajectory has been heated sufficiently, the algorithm pauses sonication in a ‘wait subtrajectory’. When temperature in one of the subtrajectories decreases below a predefined range, the algorithm is able to heat that subtrajectory specifically.
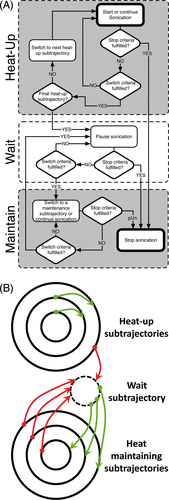
Figure 4. (A) The mean temperature within an 8 mm treatment cell over a 10 min sonication + additional 5 min monitoring in vivo. Uncorrected (grey line) and corrected (black line) temperatures clearly demonstrate the effect of B0 magnetic field drift. (B) The total baseline temperature drift over 15 min from the same sonication as in A resulted in a change of 3°C.
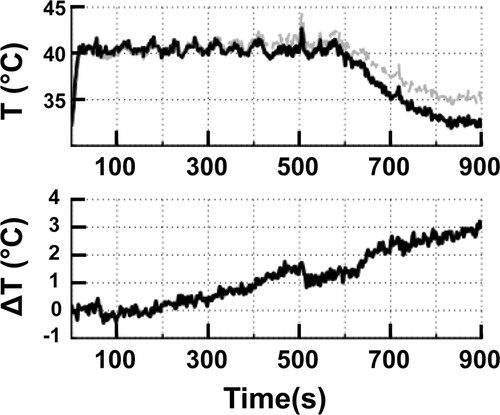
Figure 5. (A) Time-averaged spatial temperature distribution for a 12 mm treatment cell in silico (coronal plane) for 10 min mild hyperthermia with normal perfusion (1 mL/mL/min). Treatment cell is outlined in black dashed line.(B, C) Simulated mean temperature along 4, 8 and 12 mm subtrajectories at two different perfusion levels (B, normal perfusion (1 mL/mL/min) and C, high perfusion (2 mL/mL/min). Only at high perfusion level was it necessary to heat subtrajectories other than 12 mm after initial heat-up – notice the heating of the 4 mm subtrajectory at 80, 120 and 170 s (marked with asterisks in C).
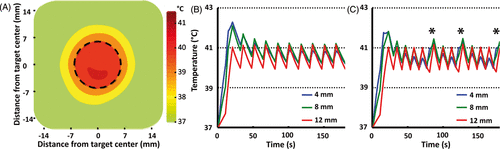
Table II. Summary of in vitro sonication results. All sonications were performed at a fixed acoustic power of 25 W. The 3D spatial offset was significantly greater than 0 for 4, 8 and 16 mm treatment cells (p = 0.0064, p = 0.0401 and p = 0.0226, respectively, one-sample t-test), but not the 12 mm treatment cell (p = 0.0963, one-sample t-test). Target mean temperature range was 40–41°C. All values are mean ± SD.
Figure 6. (A) Representative examples of mean (solid), T10, and T90 (dashed) temperatures within an 8 mm treatment cell over a 10 min sonication in vivo. Target temperature range is indicated as a grey box. (B) Representative examples of time-averaged mean temperature radial line profiles centred on the treatment cell for 4 mm, 8 mm, and 12 mm treatment cells.
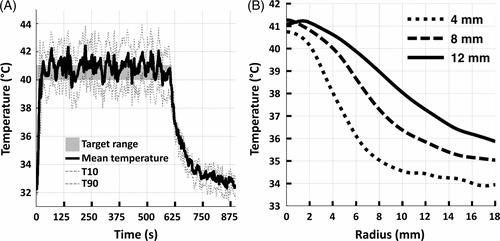
Figure 7. Representative examples of time-averaged spatial temperature distributions for 4 mm, 8 mm, and 12 mm treatment cells in vivo (coronal plane). The treatment cell is outlined in black dashed line.
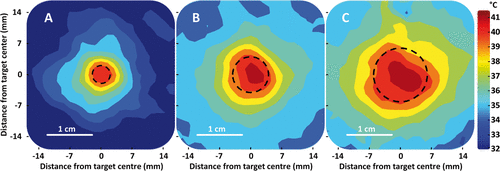
Table III. Summary of in vivo sonication results. Target mean temperature range was 40–41°C. The 3D spatial offset was significantly greater than 0 for the 4 mm and 8 mm treatment cells (p = 0.0002 and p = 0.0018, respectively, one-sample t-test), but not for the 12 mm treatment cell (p = 0.1540, one-sample t-test).
Table IV. Summary of sonication duration in different subtrajectories (as percentages of total treatment time), sonication energies, and sonication efficiencies in vivo. Energies and efficiencies are reported as mean ± SD.
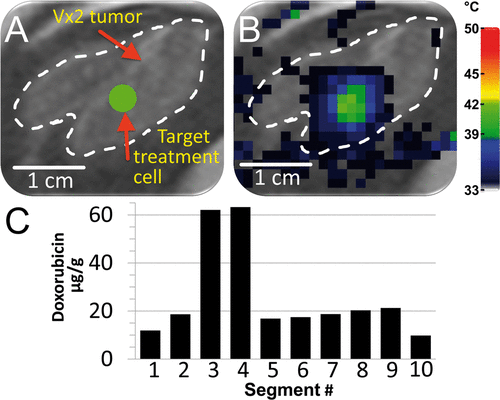
Figure 8. Demonstration of heterogeneous drug delivery. (A) VX2 tumour was clearly identified (white dashed line) on the proton density-weighted planning images and a target region within the tumour was chosen (green circle). (B) Temperature maps (colour scale) overlaid on planning images (greyscale) during a mild hyperthermia treatment with a 4 mm treatment cell, showing typical temperature distribution after 1 min of heating. (C) Doxorubicin concentration in tumour segments was determined by HPLC. Note the higher drug concentration in segments 3 and 4.