Figures & data
Figure 1. Double-strand break repair by NHEJ and HR. (A) Schematic of NHEJ-mediated DSB repair. DSB ends are recognised by the Ku70/80 heterodimer, resulting in the activation of DNA-PKcs and XRCC4/DNA ligase IV. Processing of DNA ends that cannot be directly ligated (represented by the red star) can be performed by nucleases (Artemis) and DNA polymerases, as well as polynucleotide kinase (not shown). (B) Schematic of HR-mediated DSB repair. DNA ends are organised by the Rad50/MRE11/NBS1 complex, converted by nucleolytic processing to RPA-coated single-strand DNA tails. Mediator proteins such as BRCA2 are involved in loading the core HR protein, Rad51, onto single-strand DNA generating a nucleoprotein filament capable of performing homology recognition on the template DNA. Rad54, another mediator processes Rad51-bound to double-strand DNA such that the intermediate can be handed off to a DNA polymerase to initiate DNA replication (represented by black arrows), which is as an obligatory subsequent step on the way to repair of the DSB.
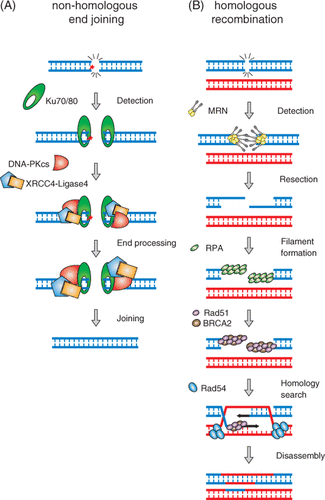
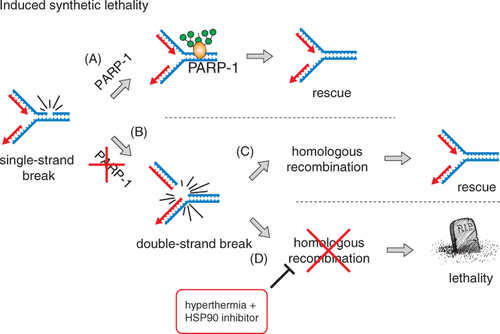
Figure 2. Induced synthetic lethality. (A) PARP-1 is important for proper repair of SSBs that occur spontaneously as a consequence of normal cellular metabolism. (B) In the absence of PARP-1 activity, unrepaired SSBs that are encountered by progressing DNA replication forks in S-phase cells are converted to DSBs. This type of DNA lesion is very cytotoxic, as even a single unrepaired DSB can result in cell death. (C) In S-phase, replication-associated DSBs are restored by HR, which involves proteins such as BRCA1/2 and Rad51. Therefore, in repair-competent cells the DSBs arising at the replication fork (due to inhibition of PARP-1) are promptly and accurately repaired by HR, with no further cytotoxic consequences. (D) However, in the absence of HR, either due to genetic mutation or induced by the hyperthermia (and heat-shock protein inhibition), these DSBs are not timely repaired and cause cell death.