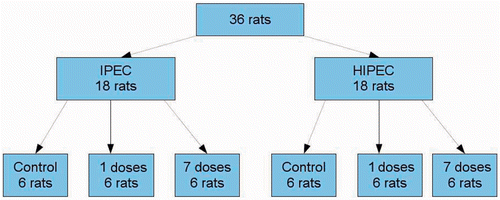
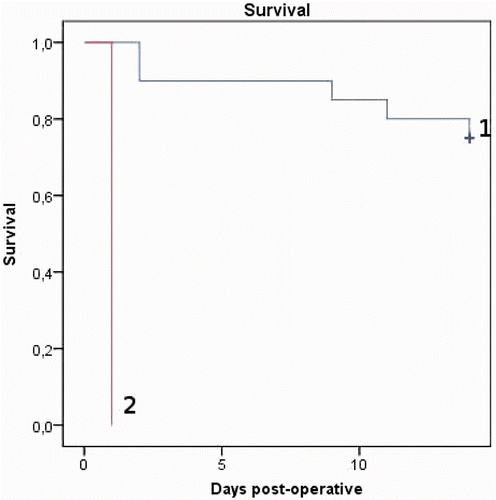
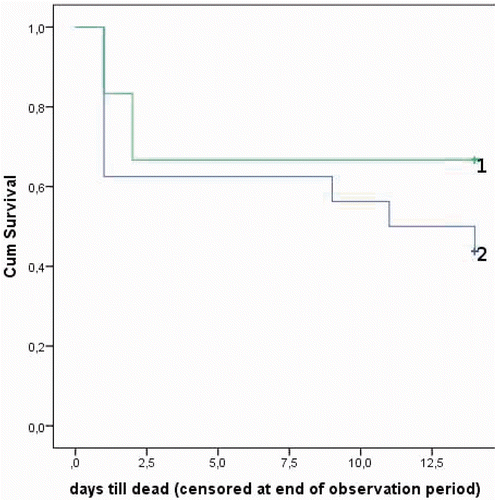
Figures & data
Figure 1. Schematic presentation of permeation process in IPEC. The drug (big circles) will move from the perfusate to the tumour, passing cell membranes (dotted line), due to the concentration difference (bold arrow). High pressure in the tumour decreases the rate of penetration, due to the lower pressure gradient between perfusate and tumour, as compared to normal tissue.
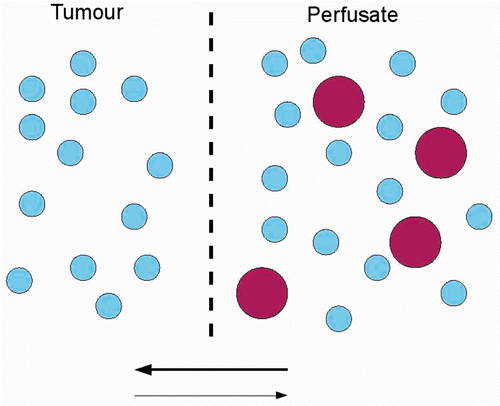
Table I. Results of IFP measurements. IFP at start: IFP at the moment the perfusate was administered in the abdominal cave (time = 0 min). IFP difference: IFP at time = 60 min minus IFP at time = 0 min. (1) Normothermic, without bevacizumab; (2) Hyperthermic, without bevacizumab; (3) Normothermic, 1 dose of bevacizumab; (4) Hyperthermic, 1 dose of bevacizumab; (5) Normothermic, 7 doses of bevacizumab; (6) Hyperthermic, 7 doses of bevacizumab.
Figure 3. Results of LA-ICP-MS. Upper left: normothermic without bevacizumab (63.75%); upper right: hyperthermic without bevacizumab (93.53%); under left: normothermic with bevacizumab (43.18%); under right: hyperthermic with bevacizumab (45.18%). Blue line: tumour border. Coloured parts: platinum concentration from 0.1 µg/g (purple) upwards.
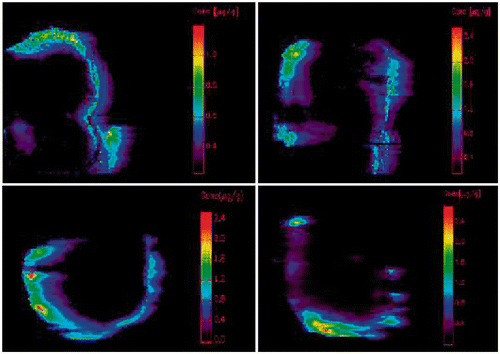
Table II. Effects of temperature on bio-availability. AUC ratio = AUC blood/AUC perfusate.
Table III. Effects of bevacizumab.
Table IV. Tumour volumes and changes.