Figures & data
Figure 1. Graph of results from gel phantom experiments reacting either AcCl or Ac2O solutions at 5 mol/L with NaOH solutions of equal concentrations. A thermocouple was placed in the reaction chamber to record the degree and duration of the temperature excursions as the reactions took place. Subsequent experiments were performed with AcCl due to the lower reactivity of Ac2O.
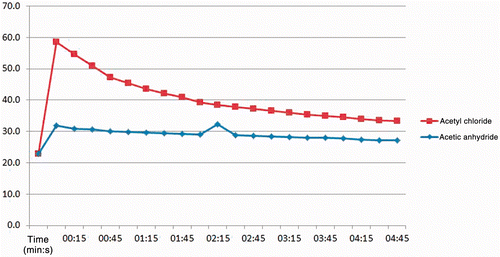
Figure 2. Temperature changes recorded over time from reaction of AcCl solutions in liver tissue with thermocouple inserted immediately adjacent and to the same depth. Concentrations ranged from 1–4 mol/L in diglyme as the solvent. Injection rate was 1 mL/min with total volume in each case of 1 mL of solution. Peak temperature using the 4 mol/L solution was 54°C, or an increase of 30°C within seconds after initiating the injection. Experiments at higher concentrations were abandoned due to the pronounced reactivity of the reagent.
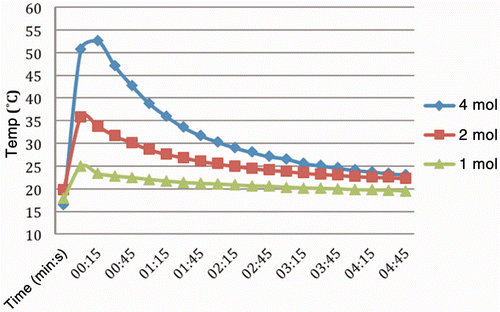
Figure 3. Change in pH in tissues over time, sectioned after injection of 750 µL solutions of AcCl (concentration range1–4 mol/L). Note in each case the initial drop is followed by a recovery to some degree. The maximum decrease was measured at 2.25, which over time rose to just above 3.
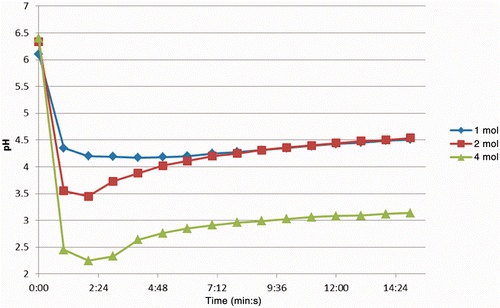
Figure 4. Relationship of coagulation volume to injection volume and concentration with AcCl solutions. The injected volume ranged from 250–2000 µL at concentrations of 1–4 mol/L. There is some variation but in general the coagulation volume increases as both variables (injection volume and concentration) are increased.
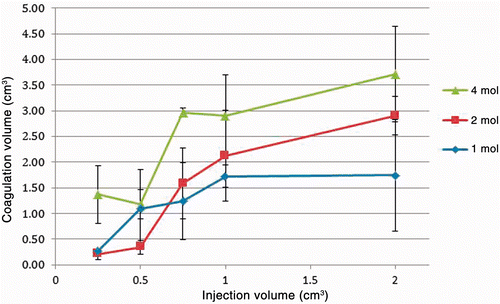
Figure 5. Infrared image captured immediately after completion of injection and bisection of specimen. Concentration was 4 mol/L with injection volume of 750 µL. Baseline tissue temperature is below 24°C and central portion of injection is at the peak of the scale, 55°C. A warmed US quarter dollar coin (24 mm diameter) was included in the same focal plane to serve as a size reference.
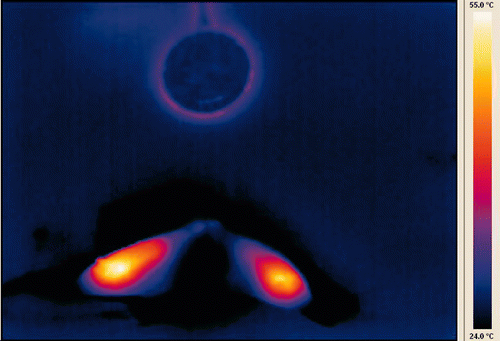
Figure 6. Gross specimen of thermochemical ablation by AcCl corresponding to infrared image in previous figure. Tan discoloration of coagulated tissue is evident throughout the treated area and extends beyond the thermal excursion shown in the infrared image.
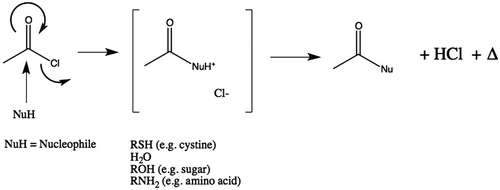
Figure 7. Generalised reaction scheme illustrating release of heat during hydrolysis of AcCl and generation of acetic acid. Where the nucleophile (Nu) is water, two equivalents of acid are produced (one each of acetic acid and hydrochloric acid). In those cases where reagent could react with tissue instead of water, only one equivalent of acetic acid would be produced. However, in those cases this implies that covalent modification of the substrate has occurred.