Figures & data
Figure 1. Polymorphonuclear leukocyte counts. Symbols and abbreviations: Δ patients; ○ healthy control subjects; ![]()
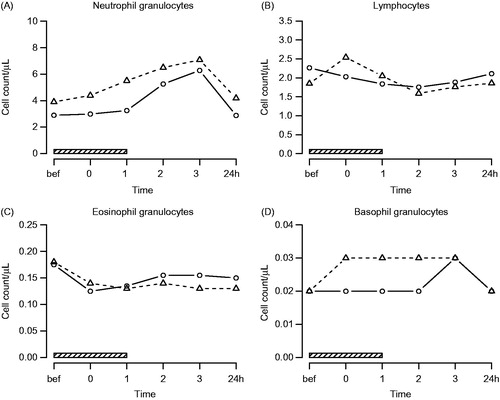
Figure 2. Lymphocyte subsets. Symbols and abbreviations: Δ patients; ○ healthy control subjects; ![]()
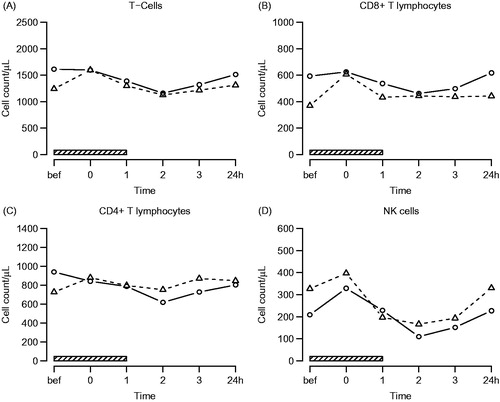
Table 1. Effects of hyperthermia on polymorphonuclear leucocytes and lymphocyte subsets (q-values).
Figure 3. TLR-4, IL-10 and HSPB1 gene expression. Symbols and abbreviations: Δ patients; ○ healthy control subjects; ![]()
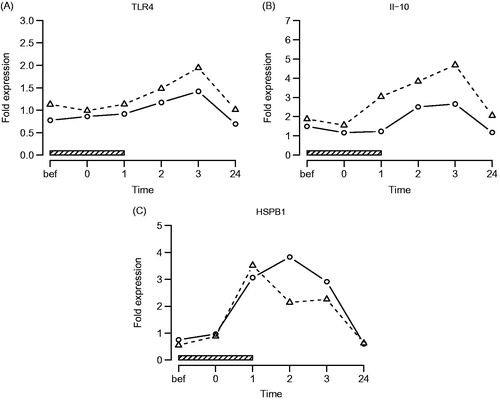
Table 2. Effects of hyperthermia treatment on TLR-4, IL-10 and HSPB1 gene expression (q-values).
Figure 4. Systemic cytokines. Symbols and abbreviations: Δ patients; ○ healthy control subjects; ![]()
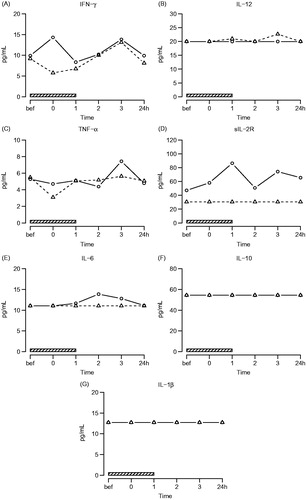
Table 3. Effects of hyperthermia treatment on plasma cytokine concentrations (q-values).
Figure 5. Acute phase reactants. Symbols and abbreviations: Δ patients; ○ healthy control subjects; ![]()
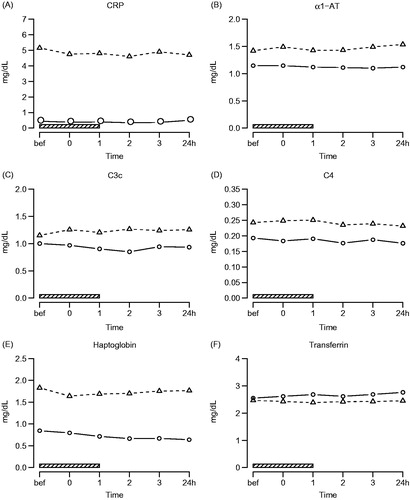
Table 4. Effects of hyperthermia treatment on acute phase reactants (q-values).
