Figures & data
Figure 1. Heat shock (42–43 °C) alters the cellular balance between pro-apoptosis and anti-apoptosis Bcl-2 family proteins: protective role of thermotolerance (40 °C). Non-thermotolerant (3 h at 37 °C) and thermotolerant (3 h at 40 °C) cells were exposed to heat shock (42–43 °C) for 3 h. (a) Western blots for protein expression in whole cell lysates are representative of at least three independent experiments. Means and SEM are shown for densitometric analysis of proteins (b) Bcl-2, (c) Bcl-xL, (d) Bax, (e) Bak, (f) Puma, (g) Noxa. Protein expression in thermotolerant and non-thermotolerant cells was normalised to GAPDH loading controls and is relative to non-thermotolerant controls at 37 °C (100%). For significant differences between heated (42–43 °C) cells and the control (37 °C), p < 0.05 (*), p < 0.001 (**). For significant differences between thermotolerant and non-thermotolerant cells at each specific temperature, p < 0.05 (#), p < 0.001 (##).
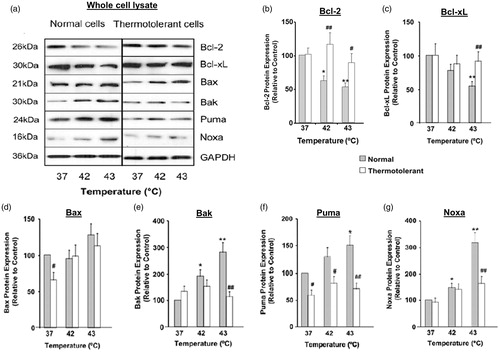
Figure 2. Heat shock (42–43 °C) causes relocalisation of Bax, Bak, Bim, Puma and Noxa to mitochondria while expression of Bcl-2 and Bcl-xL decreases: reversal by thermotolerance (40 °C). Non-thermotolerant (3 h at 37 °C) or thermotolerant (3 h at 40 °C) cells were heated (42–43 °C) for 3 h. (a) Western blots for protein expression in mitochondrial fractions are representative of at least three independent experiments. (B) The purity of cytosolic and mitochondrial fractions was confirmed using GST-π1 and cytochrome c oxidase (Cox2) antibodies, respectively. The average purity of the cytosolic fraction from at least four separate experiments was 89.72 ± 1.49% and that of the mitochondrial fraction was 92.33 ± 2.57%. Means and SEM are shown for densitometric analysis of (c) Bcl-2, (d) Bcl-xL, (e) Bax, (f) Bak, (g) Puma, (h) Noxa. Protein expression is relative to non-thermotolerant controls at 37 °C (100%). For significant differences between heated (42–43 °C) cells and control (37 °C), p < 0.05 (*), p < 0.001 (**). For significant differences between thermotolerant and non-thermotolerant cells at each specific temperature, p < 0.05 (#), p < 0.001 (##).
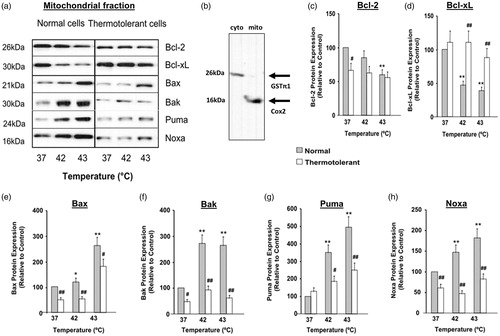
Figure 3. Decreased expression of Bcl-2 family proteins in cytosolic fractions during heat shock (42–43 °C): reversal by 40 °C thermotolerance. Thermotolerant (3 h at 40 °C) and non-thermotolerant (3 h at 37 °C) cells were heated (42–43 °C) for 3 h. (a) Western blots for protein expression in cytosolic fractions are representative of at least three independent experiments. + is a positive control for Bcl-2 expression in whole cell lysates. Means and SEM are shown for densitometric analysis of (b) Bcl-xL, (c) Bax, (d) Puma, (E) Noxa. Protein expression is relative to non-thermotolerant controls at 37 °C (100%). For significant differences between hyperthermia-treated (42–43 °C) cells and the control (37 °C), p < 0.05 (*), p < 0.001 (**). For significant differences between thermotolerant and non-thermotolerant cells at each specific temperature, p < 0.05 (#), p < 0.001 (##).
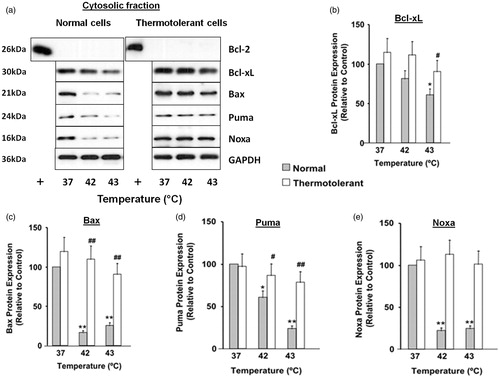
Figure 4. Hyperthermia-induced down-regulation of Bcl-2 involves ROS, and up-regulation of Puma and Noxa involves p53 and ROS. Cells were pretreated with PEG-catalase (PC) or pifithrin-α (Pα) and then heated (43 °C) for 3 h. (a) Western blots for protein expression in whole cell lysates are representative of at least three independent experiments. Means and SEM are shown for densitometric analysis of proteins: (b) Bcl-2, (c) Bax, (d) Bak, (e) Puma, (f) Noxa. Protein expression was normalised to GAPDH loading controls and is relative to non-treated controls at 37 °C. For significant differences between heated (43 °C) cells and the control (37 °C), p < 0.05 (*). For significant differences between treated and non-treated cells at each specific temperature, p < 0.05 (#).
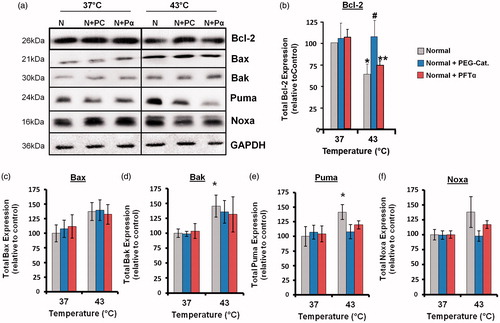
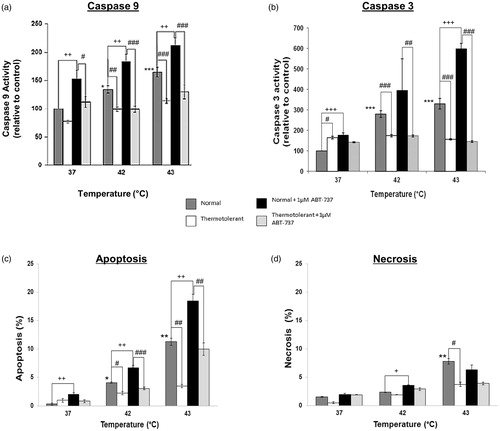
Figure 5. Bcl-2 inhibitor ATB-737 enhances hyperthermia-induced apoptosis: protective role of mild thermotolerance (40 °C). Relative activities of caspase-9 (a) and caspase-3 (b), and levels of apoptosis (c) and (d) necrosis in thermotolerant (3 h at 40 °C) and non-thermotolerant (3 h at 37 °C) cells, with or without pretreatment with ABT-737 (1 µM). Means ± SEM are from at least three independent experiments. For significant differences between heated (42–43 °C) cells and the non-thermotolerant control (37 °C), p < 0.05 (*), p < 0.001 (**). For significant differences between thermotolerant and non-thermotolerant cells at each temperature, p < 0.05 (#), p < 0.001 (##). For cells with or without ABT-737, p < 0.05 (+), p < 0.01 (++), p < 0.001 (+++).
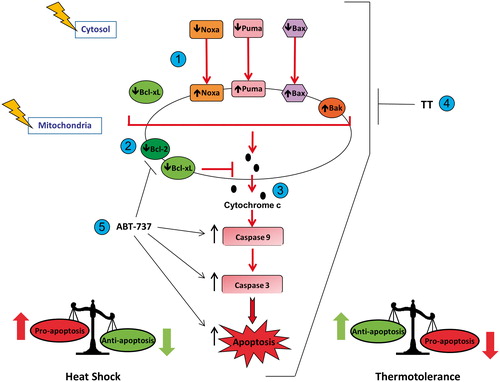
Scheme 1. Heat shock (42–43 °C) alters the pro-apoptosis/anti-apoptosis balance in Bcl-2 family proteins during apoptosis in HeLa cells. (1) Heat shock induced translocation of Bax, Puma and Noxa to mitochondria. (2) Heat shock decreased levels of Bcl-2 and Bcl-xL in mitochondria. (3) Heat shock increased the pro-apoptosis/anti-apoptosis balance in Bcl-2 family proteins in mitochondria, which led to cytochrome c release, activation of caspase-9 and caspase-3, and execution of apoptosis. (4) Thermotolerance (TT) at 40 °C decreased the translocation of Bax, Noxa and Puma to mitochondria, and inhibited cytochrome c release, caspase activation and apoptosis. (5) Inhibition of the anti-apoptotic proteins Bcl-2 and Bcl-xL by ABT-737, preventing them from interfering with heat shock-induced apoptosis.