Figures & data
Figure 1. Study design. Rabbits bearing Vx2 thigh tumours were randomised into groups that received either 1.67 mg/kg of TLD alone, or 1.67 mg/kg of TLD plus mild hyperthermia using a clinical MR-HIFU system. Tumour volume was followed on contrast enhanced T1-weighted MRI; body weight and blood counts were monitored for toxicity. Sac, sacrificed; TLD, thermosensitive liposomal doxorubicin.
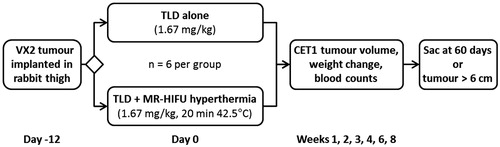
Figure 2. Experimental set-up for mild hyperthermia in rabbit Vx2 tumours using a clinical MR-HIFU system. Left: Axial survey image of a rabbit on top of a water-filled animal adaptor. A waterproofed receive-only imaging coil fits around the lower leg. The bottom film of the animal adaptor is coupled to the window of the clinical HIFU system by a gel pad; the HIFU transducer is in the oil bath below. Overlays indicate the relative size of the ultrasound beam path (dashed) and treatment cell (shaded). Right: Rendering of the animal adaptor designed for the clinical HIFU system. The detachable lid (A) is a polyimide film glued to an acrylic ring. The cylindrical water bath (B) is a 3D-printed shell that holds a volume of degassed water, which is heated by water pumped through a coiled channel printed into the walls of the cylinder. Polyimide film (C) forms the base.
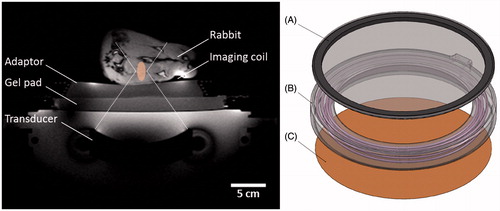
Table 1. MR imaging parameters used in this study.
Figure 3. Mild hyperthermia treatment planning in a rabbit Vx2 thigh tumour using a clinical MR-HIFU system. Three-dimensional reconstruction of contrast-enhanced T1-weighted images acquired for mild hyperthermia treatment planning. Ultrasound beam and treatment cell overlays used to guide translation and rotation of transducer to avoid heating skin, bone, and sensitive structures. For figure clarity, semi-transparent ultrasound beam overlays shown by the treatment planning software are replaced here with dotted outlines. Treatment cell overlays are shaded.
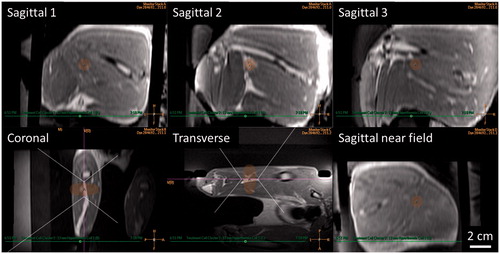
Figure 4. MRI-controlled mild hyperthermia of a Vx2 tumour in a rabbit thigh using a clinical MR-HIFU system. Top: MR temperature maps acquired after 15 min of heating. The slice labelled Sagittal 2 is centred at the focus on the tumour. HIFU beam path is outlined in white, imaging slice locations are indicated in pink. High temperature measurements in the water outside of the rabbit thigh are the result of imaging artefacts. Bottom: Plot of median temperatures vs. time in three sagittal slices across the treatment cell. The range between T10 and T90 temperatures in the middle slice is shaded. Vertical lines indicate the start and end of thermosensitive liposomal doxorubicin (TLD) infusion, and end of sonication.
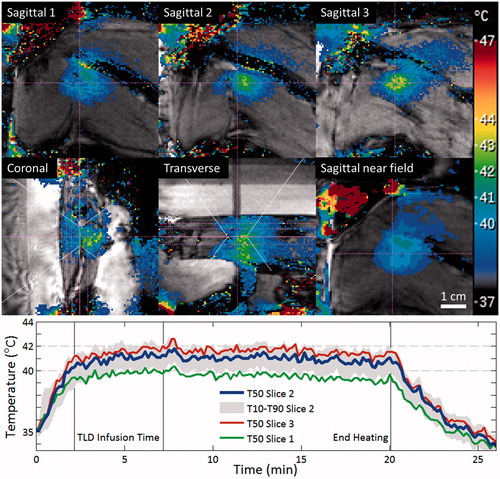
Table 2. MR-HIFU mild hyperthermia of rabbit Vx2 tumours.
Figure 5. Rabbit Vx2 tumour response after hyperthermia-mediated drug delivery using thermosensitive liposomal doxorubicin and MR-HIFU. Contrast-enhanced T1-weighted images from treatment planning and 1, 2, and 3 weeks post-treatment. Axial images through the 3D volume are shown at the location of the maximal tumour extent. Left: Rabbits treated with TLD alone. Right: TLD plus MR-HIFU mild hyperthermia. Scale bar = 2 cm. TLD, thermosensitive liposomal doxorubicin.
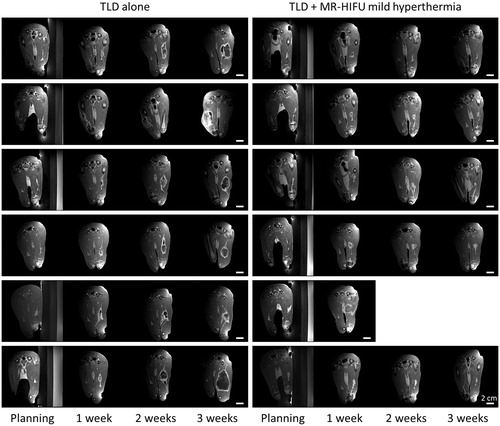
Figure 6. Effect of TLD with or without MR-HIFU mild hyperthermia in rabbit Vx2 tumours. (A) Tumour volume calculated from 3D contrast-enhanced T1-weighted MR images. Inset shows the volume at the time of treatment. (B) Overall survival. All rabbits in the TLD group reached the end point of maximum tumour dimension exceeding 6 cm. One rabbit in the TLD + MR-HIFU group experienced severe toxicity and was sacrificed at 7 days, one reached tumour size end point at 53 days, the other four survived to the end of the study. TLD, thermosensitive liposomal doxorubicin.
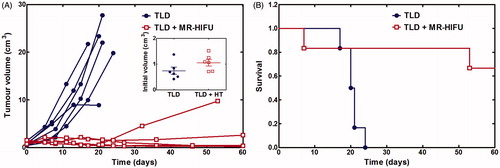
Table 3. Tumour volume grouped by week.
Figure 7. Subgroup analysis among rabbits treated with thermosensitive liposomal doxorubicin plus MR-HIFU mild hyperthermia. Strong response: rabbits with smaller tumour volume at the 60-day study end point than at day 0. Weak response: rabbits with tumour relapse (tumour size end point before day 60, or larger tumour size at day 60 than at day 0) or acute toxicity (sacrificed prior to day 60). The weak response rabbit labelled ‘motion’ had short duration of heating above 40 °C, the rabbit labelled ‘large tumour’ had a large initial tumour size, and the rabbit labelled ‘toxicity’ was sacrificed early due to acute treatment-related toxicity attributable to high thermal dose.
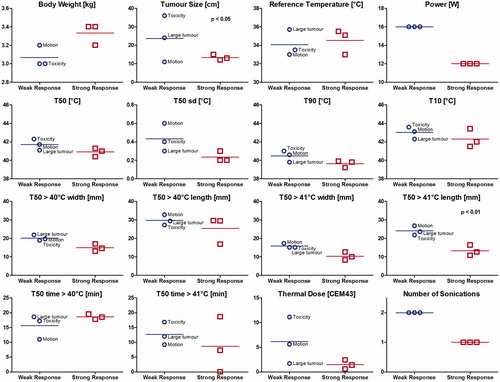
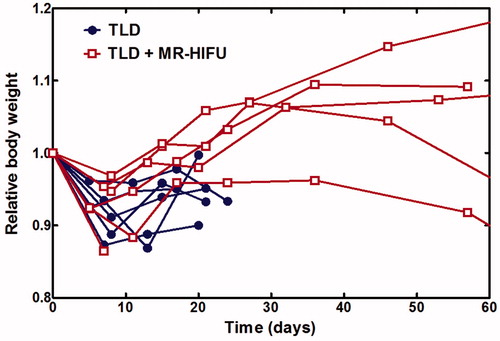
Figure 8. Relative body weight over time. All rabbits experienced an initial decrease in body weight and subsequent recovery. TLD, thermosensitive liposomal doxorubicin.