Figures & data
Figure 1. Overview of the tumour microenvironment. The upper boxes contain symbols for cell types found in tumours, along with factors that influence the tumour microenvironment. Many cell types are found, including macrophages, lymphocytes, fibroblasts, and vascular endothelial cells. Cross talk between these cell types promotes tumour growth. The lower box summarises how the microenvironment changes in response to hyperthermia. Under baseline conditions (left), tumours contain a variety of different cell types. Pleiotropic tumour cell phenotypes include stem cells, cells exhibiting epithelial or mesenchymal characteristics, and cells undergoing autophagy. Oxidative stress and inflammation then influence which phenotype predominates following hyperthermia treatment. Changes induced by hyperthermia include (1) altering cytokine and chemokine levels, (2) promoting tumour cell survival, (3) driving epithelial cells toward mesenchymal (invasive) phenotype, (4) promoting metastasis, and (5) altering immunogenicity of the remaining tumour cells.
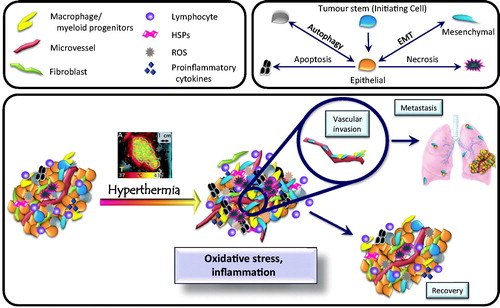
Figure 2. Depiction of the number of publications focusing on the two subjects ‘tumour’ and ‘microenvironment’ since the year 2000. The number of citations has increased to match the citations. The cumulative number of citations focusing on these two MeSH terms is over 500,000. In this review we consider how well the field of hyperthermia has integrated into this emerging hallmark and the opportunities that exist to further the impact of hyperthermia in defining the field of tumour microenvironment.
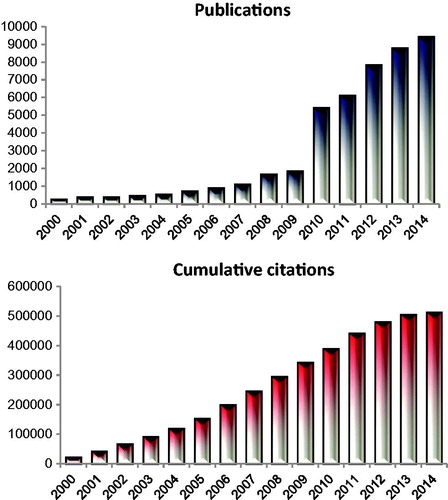
Figure 3. Hyperthermia-mediated cell killing is enhanced by 150 μM lonidamine. Lonidamine was administered beginning 1 h prior to a 2-h hyperthermia treatment. Cells were grown and treated at the indicated extracellular pH (pHe). Hyperthermia increased cell killing at pHe of both 7.3 and 6.7. Lonidamine had a synergistic effect on cell killing in cells grown at pHe 6.7 only. (Data re-plotted from Coss et al. [Citation14]).
![Figure 3. Hyperthermia-mediated cell killing is enhanced by 150 μM lonidamine. Lonidamine was administered beginning 1 h prior to a 2-h hyperthermia treatment. Cells were grown and treated at the indicated extracellular pH (pHe). Hyperthermia increased cell killing at pHe of both 7.3 and 6.7. Lonidamine had a synergistic effect on cell killing in cells grown at pHe 6.7 only. (Data re-plotted from Coss et al. [Citation14]).](/cms/asset/ecaeed3b-bf2e-4e4f-82e3-897b019fd06e/ihyt_a_1091093_f0003_c.jpg)
Figure 4. Hyperthermia augments cytotoxic T cell killing when applied in conjunction with a recombinant hyper-IL-6 fusion protein (H-IL-6). H-IL-6 is comprised of IL-6 and s-IL-6R. When combined with hyperthermia, this treatment promotes CD8+ T cell extravasation through the tumour bed, increasing tumour cell death. (Adapted from Fisher et al. [Citation52]).
![Figure 4. Hyperthermia augments cytotoxic T cell killing when applied in conjunction with a recombinant hyper-IL-6 fusion protein (H-IL-6). H-IL-6 is comprised of IL-6 and s-IL-6R. When combined with hyperthermia, this treatment promotes CD8+ T cell extravasation through the tumour bed, increasing tumour cell death. (Adapted from Fisher et al. [Citation52]).](/cms/asset/601c470c-2dd3-486c-82f7-2f8e787b0137/ihyt_a_1091093_f0004_c.jpg)
Figure 5. Hyperthermia induced upregulation of HIF-1 in tumours. 4T1 mouse mammary tumours were grown in the flanks of mice. The tumour-bearing leg was heated at 34 °C or 42 °C for 1 h. HIF-1 expression was assessed by a luciferase reporter gene at various time points post-treatment. Luciferase activity was normalised to tumour volume at the time of treatment. Data points represent mean ± sem; n = 4–8/group. *p < 0.01. (Data re-plotted from Moon et al. [Citation76].) HT, hyperthermia.
![Figure 5. Hyperthermia induced upregulation of HIF-1 in tumours. 4T1 mouse mammary tumours were grown in the flanks of mice. The tumour-bearing leg was heated at 34 °C or 42 °C for 1 h. HIF-1 expression was assessed by a luciferase reporter gene at various time points post-treatment. Luciferase activity was normalised to tumour volume at the time of treatment. Data points represent mean ± sem; n = 4–8/group. *p < 0.01. (Data re-plotted from Moon et al. [Citation76].) HT, hyperthermia.](/cms/asset/41d26303-0ea4-43b1-9da0-34d0428781af/ihyt_a_1091093_f0005_c.jpg)
Figure 6. Hyperthermia increases oxygenation via modulation of HIF-1 transcriptional activity. Moon et al. [Citation76] demonstrated that hyperthermia activates the ERK pathway, which leads to up-regulation of NADPH oxidase. NADPH activity increases intracellular superoxide anions, which then stimulates HIF-1 stabilisation. Reoxygenation follows as surviving tumour cells reduce oxygen consumption rate as they switch to aerobic glycolysis. In concert, hyperthermia decreases the mitochondrial membrane potential to further lower oxygen consumption and improve overall oxygenation levels.
![Figure 6. Hyperthermia increases oxygenation via modulation of HIF-1 transcriptional activity. Moon et al. [Citation76] demonstrated that hyperthermia activates the ERK pathway, which leads to up-regulation of NADPH oxidase. NADPH activity increases intracellular superoxide anions, which then stimulates HIF-1 stabilisation. Reoxygenation follows as surviving tumour cells reduce oxygen consumption rate as they switch to aerobic glycolysis. In concert, hyperthermia decreases the mitochondrial membrane potential to further lower oxygen consumption and improve overall oxygenation levels.](/cms/asset/4535f0b4-3b3f-46f0-8713-6c335b580c69/ihyt_a_1091093_f0006_c.jpg)
