Figures & data
Figure 1. Cell death and release of danger signals of melanoma cells after irradiation and hyperthermia. The cell death forms of B16-F10 melanoma cells were analysed with two-colour flow cytometry after staining of the cell suspension with AnxA5-FITC and PI, 24 h after treatment with RT (2 Gy), HT (41.5 °C for 1 h) or a combination of both. The percentage of apoptotic cells (positive for AnxA5 binding and negative for PI staining) is displayed in A, and that of necrotic cells (positive for both, AnxA5 binding and PI staining) in B. The fold increase of HMGB1 (C) and Hsp70 (D) compared to mock controls in the supernatants of the tumour cells was determined with the ELISA technique after the respective treatments. Representative data of one out of three experiments, each performed in triplicate (A, B) or duplicate (C, D), are presented as mean ± SD. *p < 0.05; **p < 0.01.
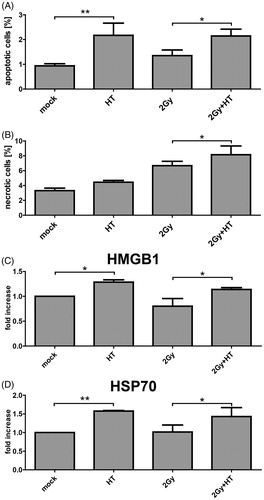
Figure 2. Establishment of local treatment procedures for radiotherapy and hyperthermia of tumour-bearing mice. To locally irradiate the B16-F10 tumour -bearing C57/BL6 mice, a Plexiglas® box was manufactured which allows the irradiation of three mice at once (A). The mice were anaesthetised before being placed in the box. For the irradiation procedure, the mice were kept under isoflurane anaesthesia to avoid them moving. The planning of the irradiation was conducted using a computer tomography image of the irradiation box and tumour-bearing mice (not displayed) with Philips pinnacle software to obtain an optimal target volume. Afterwards the dosimetry of the irradiation was performed manually with a calibrated ionisation chamber. To further protect normal tissue the gantry of the 6 MV linear accelerator was rotated to 340°. The light field control of the irradiation field for the tumour-bearing mice (arrows) is displayed in the lower image of A. For local hyperthermia (B), the mice were also anaesthetised and the tumours were heated with microwave catheters to 41.5 °C for 30 min using the BSD50 hyperthermia system. On the other side of the tumour temperature was controlled with a sensor. To prevent cooling of the body, the mice were placed on a plate which was heated with warm water at 37 °C (heating plate). The body temperature of the mice was controlled with another temperature sensor. To improve the heating of the tumours, the mice were covered with aluminium foil. Both the local temperature in the tumour and the body temperature were continuously controlled.
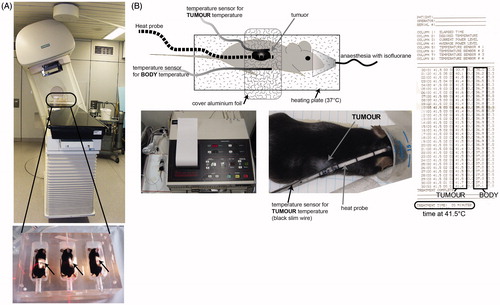
Figure 3. Impact of fractionated radiotherapy and hyperthermia on tumour volume of B16-F10 tumours in C57BL6 mice. The growth of syngeneic B16-F10 tumours in wild-type C57/BL6 mice is displayed. The tumours were locally irradiated on days 8, 9 and 10 with a clinically relevant single dose of 2 Gy using a linear accelerator. Hyperthermia (HT) was performed 4 h after irradiation on days 8 and 10. For this, the mice were heated locally under temperature control to 41.5 °C for 30 min. An electronic caliper was used to monitor the tumour volume. Joint data of three independent experiments, each with two mice per group, are presented as mean ± SD. *p < 0.05 related to mock-treated tumours.
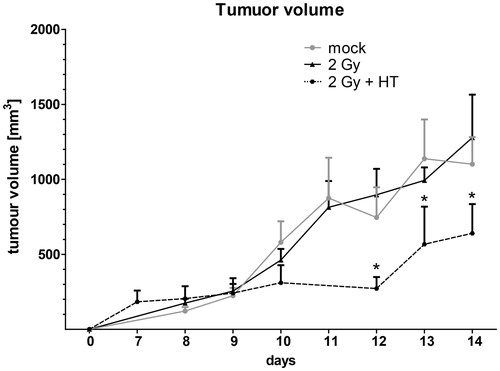
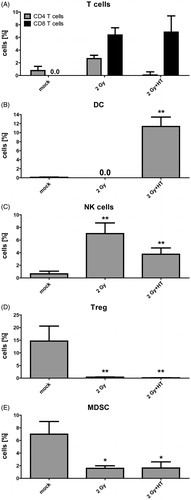
Figure 4. Impact of fractionated radiotherapy and hyperthermia on immune cell infiltration into B16-F10 tumours. (A) The infiltration of immune cells (CD4+ or CD8+ T cells), (B) dendritic cells (DC), (C) natural killer (NK) cells, (D) regulatory T cells (Treg), and (E) myeloid-derived suppressor cells (MDSC), into B16-F10 tumours on C57/BL6 mice was analysed by multicolour flow cytometry 24 h after treatment with fractionated radiotherapy (RT) with a single dose of 2 Gy and fractionated RT plus HT. Joint data of three experiments, each performed in duplicates, are presented as mean ± SD. *p < 0.05; **p < 0.01 related to mock treated tumours.