Figures & data
Figure 1. Experimental protocol. Control rats were exposed to atmospheric air throughout the same period under respective thermal conditions. Abbreviations: s - stabilisation period; r - reoxygenation period; ![]()
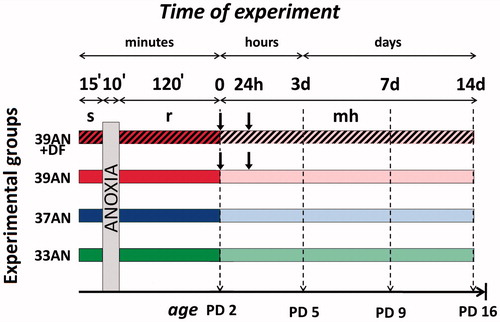
Figure 2. Effects of neonatal anoxia, neonatal body temperature (33 °C, 37 °C and 39 °C) and chelation of iron with deferoxamine (DF) on cerebral concentration of malondialdehyde (MDA) in newborn rats immediately (panel A), 3 (panel B), 7 (panel C) and 14 (panel D) days after exposure to anoxia or to control conditions. Data are presented as means ± SEM. Statistically significant differences between anoxic animals and their control counterparts at the same body temperatures are denoted: #p < 0.05 and ###p < 0.001; and those between the temperature variants of control or anoxic animals are denoted: **p < 0.01 and ***p < 0.001. Statistically significant differences between rats forced to maintain neonatal body temperature of 39 °C injected with saline and their counterparts injected with deferoxamine are denoted: ^^p < 0.01 and ^^^p < 0.001.
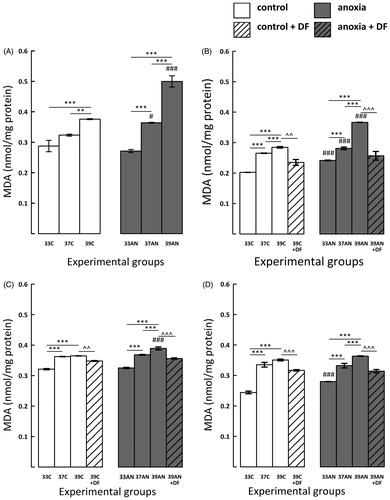
Figure 3. Effects of neonatal anoxia, neonatal body temperature (33 °C, 37 °C and 39 °C) and chelation of iron with deferoxamine (DF) on cerebral concentration of glutathione (GSH) in newborn rats immediately (panel A), 3 (panel B), 7 (panel C) and 14 (panel D) days after exposure to anoxia or to control conditions. Data are presented as means ± SEM. Statistically significant differences between anoxic animals and their control counterparts at the same body temperatures are denoted: #p < 0.05, ##p < 0.01 and ###p < 0.001; and those between the temperature variants of control or anoxic animals are denoted: *p < 0.05 and ***p < 0.001. Statistically significant differences between rats forced to maintain neonatal body temperature of 39 °C injected with saline and their counterparts injected with deferoxamine are denoted: ^p < 0.05 and ^^^p < 0.001.
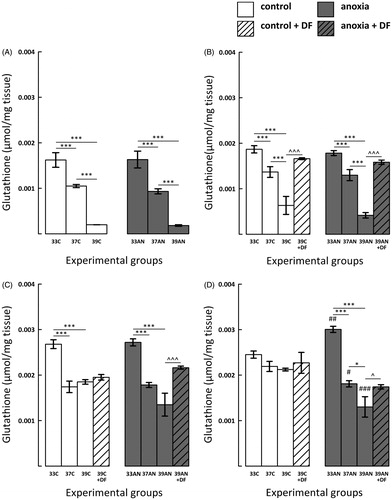
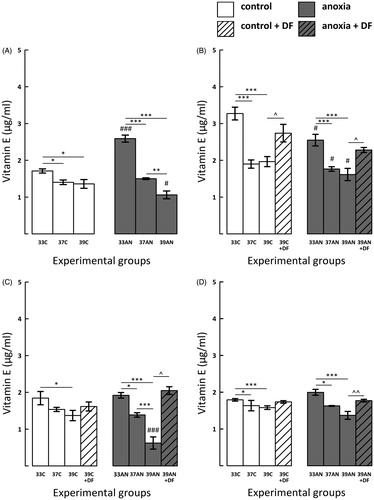
Figure 4. Effects of neonatal anoxia, neonatal body temperature (33 °C, 37 °C and 39 °C) and chelation of iron with deferoxamine (DF) on cerebral concentration of vitamin E in newborn rats immediately (panel A), 3 (panel B), 7 (panel C) and 14 (panel D) days after exposure to anoxia or to control conditions. Data are presented as means ± SEM. Statistically significant differences between anoxic animals and their control counterparts at the same body temperatures are denoted: #p < 0.05 and ###p < 0.001; and those between the temperature variants of control or anoxic animals are denoted: *p < 0.05,**p < 0.01 and ***p < 0.001. Statistically significant differences between rats forced to maintain neonatal body temperature of 39 °C injected with saline and their counterparts injected with deferoxamine are denoted: ^p < 0.05 and ^^p < 0.01.