Figures & data
Table 1. Summary of treatment parameters and measured HepG2 tumour temperatures in mice exposed to AMF.
Figure 1. BNF particle size characterisation in aqueous suspensions and as BNF-lip. (A) TEM images of BNF in aqueous suspension, and (B) cBNF-lip; (C) z-averaged values of intensity obtained from photon correlation spectroscopy (PCS). Note: images have different magnifications.
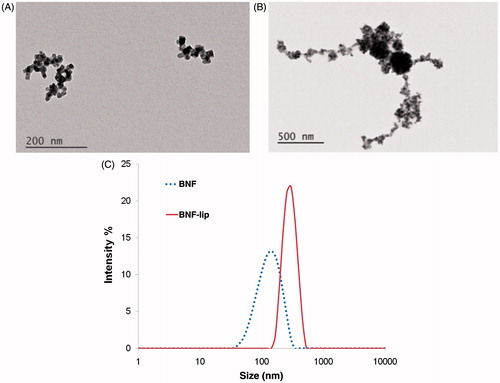
Figure 2. BNF versus BNF-lip formulation heating when exposed to AMF. (A) Direct comparison of specific heating rate of BNF with BNF-lip, (dT/dt) × (1/mg Fe), measured by estimating the slope of temperature versus time curve, where dT/dt is the slope of temperature versus time curve determined using methods reported by Bordelon et al. [Citation36]. Heating comparisons were performed at peak amplitudes 20 and 32 kA/m and 155 (± 5) kHz. (B) Specific heating rate measurements of BNF-lip formulation with varying amplitude at 155 (± 5) kHz.
![Figure 2. BNF versus BNF-lip formulation heating when exposed to AMF. (A) Direct comparison of specific heating rate of BNF with BNF-lip, (dT/dt) × (1/mg Fe), measured by estimating the slope of temperature versus time curve, where dT/dt is the slope of temperature versus time curve determined using methods reported by Bordelon et al. [Citation36]. Heating comparisons were performed at peak amplitudes 20 and 32 kA/m and 155 (± 5) kHz. (B) Specific heating rate measurements of BNF-lip formulation with varying amplitude at 155 (± 5) kHz.](/cms/asset/b7519ff2-70dc-404c-adaf-db9e4ff7243b/ihyt_a_1159737_f0002_c.jpg)
Figure 3. (A) Measured temperature change (final − initial) at the surface of HepG2 tumours following heating in AMF for group I mice (1 per cohort; see text). Tumours were injected with BNF-lip formulation at varying dose combinations (VBNF/Vtumour) and exposed to AMF with variable amplitude. Abscissa represents the injected dose, measured as the ratio of injected volume BNF suspension and measured tumour volume. Treatment duration was 15 min. (B) Mean values of tumour surface temperature plotted with time comparing heating in HepG2 tumours in group II mice following intratumoural injections of one of PBS, BNF, or BNF-lip. AMF amplitude was fixed at 20 kA/m and duration of exposure was 15 min. Treatments commenced 18 h after intratumoural injections (D = 0.5 Vtumour (mL)). (C) Mean time–temperature data comparing difference of tumour surface temperature with rectal temperatures for the cohort of mice described in B. (D) As in B; however, duration of treatment was 20 min and subject group included additional sham (AMF 0 kA/m) cohort. (E) Mean tumour–rectal temperature differences obtained from cohort described in D. Grey shading in Figures 3B and 3D are created by error bars representing the calculated standard error of mean values.
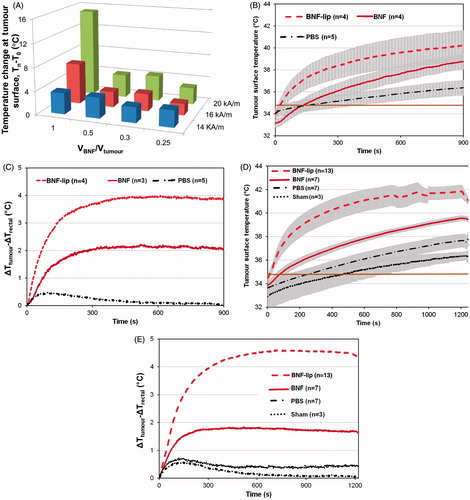
Figure 4. Representative X-ray (CT) and magnetic resonance (MR) images of rabbit liver and VX2 tumours. (A) Single-shot image of rabbit abdominal region during angiography injection of BNF-lip. (B) Axial T1 image of liver VX2 tumour in a rabbit before intra-arterial injection of BNF-lip formulation. (C) Axial T1 image of liver VX2 tumour in a rabbit at 7 days after intra-arterial BNF-lip injection. Note the pronounced paramagnetic feature of iron at the tumour site. (D) X-ray (CT) axial unenhanced image of the liver and VX2 tumour at 7 days after intra-arterial BNF-lip injection. Note the intratumoural deposition of BNF-lip (positive X-ray material in the tumour area). The arrow on each image indicates the location of the tumour.
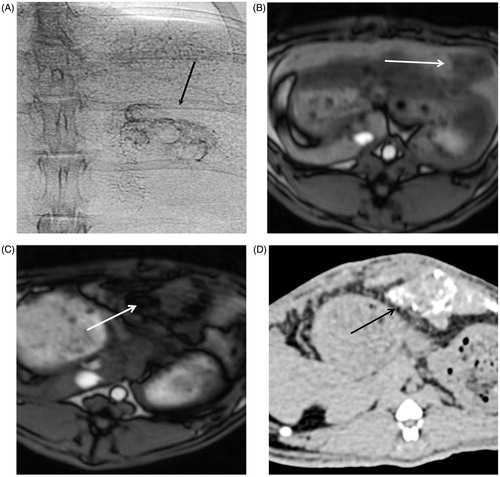
Figure 5. Histopathological images of Prussian blue-stained tissues. (A) Section of HepG2 tumour (2× magnification) harvested 3 days post-AMF–hyperthermia with BNF-lip showing necrotic cells in immediate vicinity of tumour evidenced by pale colouring, and localisation of BNF particles (blue) in the vicinity of the presumed needle track. (B) As in A, but at 8× magnification. (C) Section of liver VX2 tumour harvested at 7 days after intra-arterial injection with BNF-lip. Note that iron is deposited either in the tumour interstitium (C) and/or inside the tumour vessels (D).
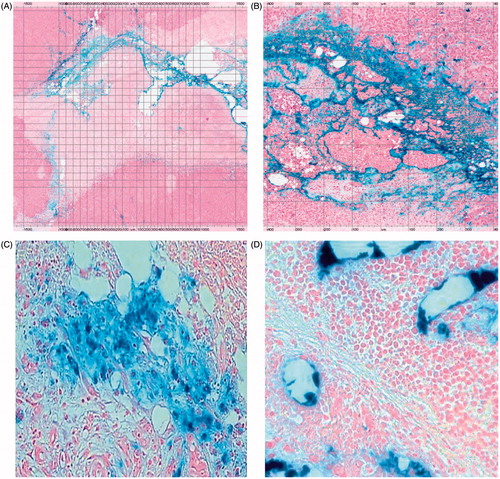
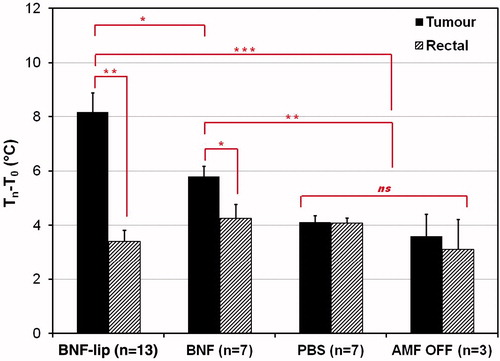
Figure 6. Mean tumour and rectal temperature changes (final − initial) measured for cohorts of mice injected with one of (D = 0.5Vtumour (ml)), PBS (saline control), BNF, or BNF-lip exposed to AMF 18 h after injection to AMF at 20 kA/m for duration of 20 min. Sham controls were injected with either PBS or BNF and placed in the AMF coil (as with the other cohorts) for 20 min with 0 kA/m amplitude. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant, p > 0.05.