Figures & data
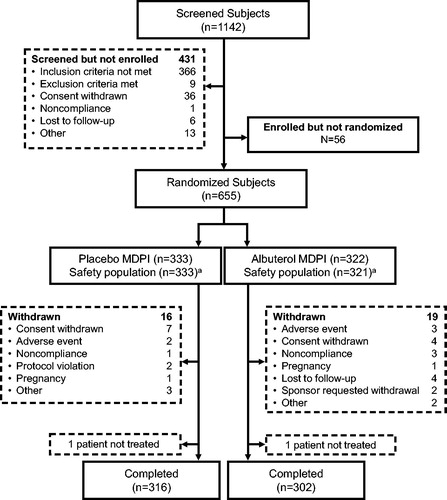
Figure 1. Study design of three pivotal phase three studies (ABS-AS-301, ABS-AS-304 and ABS-AS-307). HFA, hydrofluoroalkane; MDI, metered-dose inhaler; MDPI, multidose dry powder inhaler; PRN, as needed; QID, four times a day. aAll patients were provided with an albuterol HFA MDI to use as needed for breakthrough asthma symptoms. bFinal follow-up of adverse events was conducted 3 (±1) days after the last treatment visit.
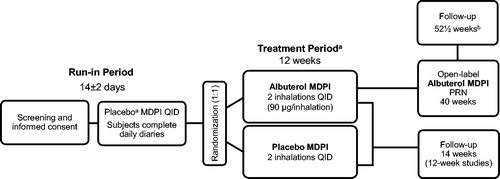
Figure 2. Patient disposition for the integrated safety population. MDPI, multidose dry powder inhaler; QID, four times a day. aOne patient in one of the 12-week double-blind studies took both albuterol MDPI and placebo MDPI in error and was therefore included in both treatment groups of the safety population. Thus, 321 patients were treated with albuterol MDPI 180 µg QID and 333 patients were treated with placebo MDPI.