Figures & data
Figure 1. Study design of the phase I part of the study. Sunitinib was initiated 1 week prior to rIL-21 exposure and consisted of 50 mg OD at a ‘4 weeks on and 2 weeks off’ schedule.
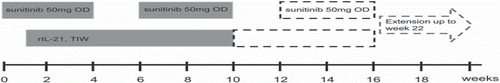
Table I. Patient characteristics.
Table II. Drug-related adverse event profile occurring in more than 30% of patients or grade 3-4 severity.