Figures & data
Figure 1. Patient flow diagram. A total of 150 eligible patients, fulfilling the inclusion criteria, were screened. One hundred accepted participation and gave informed consent. Seven patients went off study. Reasons for patients to go off-study were a) two patients refused to accept further waiting time for a second stent after failed insertion of the first stent, b) in three patients previously unknown stricture in the urethra was discovered during insertion, c) one patient had the stent removed as urine outlet was obstructed (unrecognized TURP patient), d) one patient experienced an iatrogenic displacement during an unplanned cystoscopy. In the remaining 93 patients a Memocore™ were inserted in 61 patients and a Memokath™ in 32. The stent migrated in three patients.
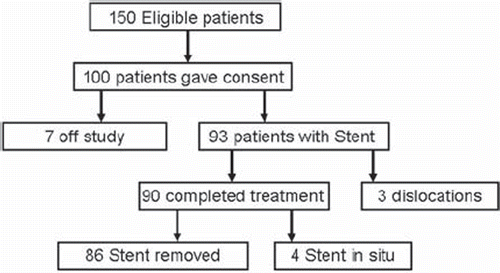
Figure 2. The memory shape Nickel-Titanium (Ni-Ti) prostate stents used as fiducial in the study. Both stents are shown in their expanded form. Both stents are made from 0.65 mm diameter Ni-Ti wire. The length of the stent is adapted to the individual length of the prostate part of urethra prior to insertion. a) The Memocore™ stent used in case the prostate part of urethra less than or equal to 40 mm. b) The Memokath™ stent used in case the prostate part of urethra of more than 40 mm.
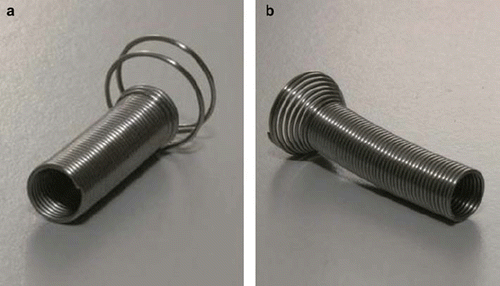
Table I. Demographics for patients treated with the stent (n = 90).
Figure 3. Plot of prostate volumes outlined on pre-radiotherapy and mid-radiotherapy MR scans. Black dots represent the three patients in whom a potential partial migration was estimated comparing sagittal MR images from the two MR scans. Other patients are represented by a white dot. a) Mid-treatment MR prostate volume (MRmid) plotted against the prostate pre-treatment volume outlined on MR (MRpre). Data are from the 90 patients in whom volumes were available in both MR scans. Error bars represents change in volume if the radius of the prostate is changed plus or minus one millimetre. b) Mismatch in MR volumes in co-registered MR volumes were defined as MRpre-MRmid and MRmid - MRpre. A corresponding theoretical difference (CAP2mm) was calculated assuming that the prostate is a sphere and the stent has partial migrated 2 mm between MR scans. Difference between the CAP2mm volume and the two mismatch volumes are plotted in Any true migration of equal to or more than 2 mm should give a dot in the grey area.
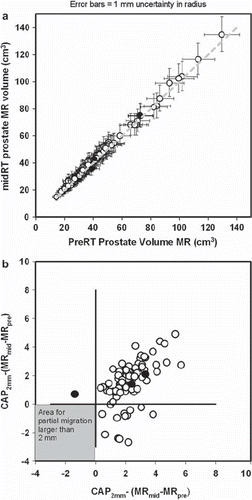
Table II. Acute toxicities related to insertion and removal of fiducials in the prostate, i.e. gold markers or prostate stent.
Table III. Acute urinary toxicities estimated using the International Prostate Symptom Score (IPSS) patient questionnaire.